Lewis Structures And Resonance Worksheet Page 2
ADVERTISEMENT
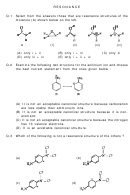
3.) Draw 3 resonance structures for SO
(no O-O bonds) – use formal charges to determine the “best”
2
structure(s) – model your set-up as question 1 is displayed
S: 1 x 6 = 6 O: 2 x 6 = 12
6 + 12 = 18 valence electrons
I
II
III
O
S
O
O
S
O
O
S
O
1
2
1
2
1
2
S: 6 – 5 = +1
S: 6 – 5 = +1
S: 6 – 6 = 0
O
: 6 – 6 = 0
O
: 6 – 7 = -1
O
: 6 – 6 = 0
1
1
1
O
: 6 – 7 = -1
O
: 6 – 6 = 0
O
: 6 – 6 = 0
2
2
2
Structure 3 is the best structure – all formal charge values are zero! Molecular shape: bent,
2
o
VSEPR: trigonal planar, hybridization: sp
angle: 120
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2