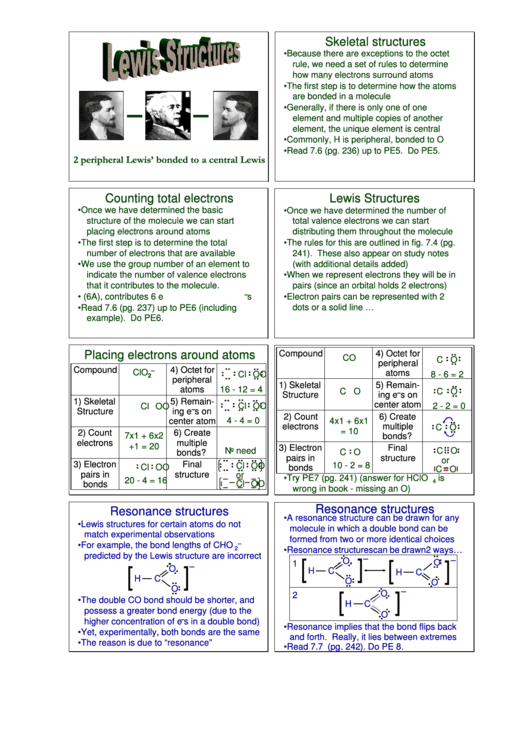
Lewis Structures
ADVERTISEMENT
Skeletal structures
Skeletal structures
• Because there are exceptions to the octet
rule, we need a set of rules to determine
how many electrons surround atoms
• The first step is to determine how the atoms
are bonded in a molecule
• Generally, if there is only one of one
element and multiple copies of another
element, the unique element is central
• Commonly, H is peripheral, bonded to O
• Read 7.6 (pg. 236) up to PE5. Do PE5.
2 peripheral Lewis’ bonded to a central Lewis
Counting total electrons
Counting total electrons
Lewis Structures
Lewis Structures
• Once we have determined the basic
• Once we have determined the number of
structure of the molecule we can start
total valence electrons we can start
placing electrons around atoms
distributing them throughout the molecule
• The first step is to determine the total
• The rules for this are outlined in fig. 7.4 (pg.
number of electrons that are available
241). These also appear on study notes
(with additional details added)
• We use the group number of an element to
indicate the number of valence electrons
• When we represent electrons they will be in
that it contributes to the molecule.
pairs (since an orbital holds 2 electrons)
–
s
• E.g. O in group VIA (6A), contributes 6 e
• Electron pairs can be represented with 2
dots or a solid line …
• Read 7.6 (pg. 237) up to PE6 (including
example). Do PE6.
Compound
4) Octet for
Placing electrons around atoms
Placing electrons around atoms
CO
C O
peripheral
Compound
4) Octet for
–
ClO
atoms
O
Cl O
8 - 6 = 2
2
peripheral
1) Skeletal
5) Remain-
16 - 12 = 4
atoms
C O
C O
–
ing e
Structure
s on
1) Skeletal
5) Remain-
center atom
O
Cl O
O
Cl O
2 - 2 = 0
–
Structure
ing e
s on
2) Count
6) Create
4 - 4 = 0
4x1 + 6x1
center atom
electrons
multiple
C O
= 10
2) Count
6) Create
7x1 + 6x2
bonds?
electrons
multiple
+1 = 20
3) Electron
Final
No need
C O
C O
bonds?
pairs in
structure
or
-
3) Electron
Final
O
Cl O
10 - 2 = 8
O
Cl O
bonds
C O
pairs in
structure
or
-
• Try PE7 (pg. 241) (answer for HClO
is
20 - 4 = 16
4
O
Cl O
bonds
wrong in book - missing an O)
Resonance structures
Resonance structures
Resonance structures
Resonance structures
• A resonance structure can be drawn for any
• Lewis structures for certain atoms do not
molecule in which a double bond can be
match experimental observations
formed from two or more identical choices
–
• For example, the bond lengths of CHO
2
• Resonance structures can be drawn 2 ways…
predicted by the Lewis structure are incorrect
[
]
[
]
–
–
O
1
[
]
–
O
H
C
H
C
H
C
O
O
[
]
–
O
2
• The double CO bond should be shorter, and
H
C
possess a greater bond energy (due to the
–
higher concentration of e
s in a double bond)
• Resonance implies that the bond flips back
• Yet, experimentally, both bonds are the same
and forth. Really, it lies between extremes
• The reason is due to “resonance”
• Read 7.7 (pg. 242). Do PE 8.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








