Example Of A Reactions That Forms Carbonic Acid, Then Gas: The "Baking Soda Volcano" Page 2
ADVERTISEMENT
122
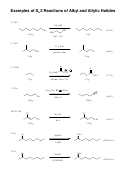
A few more double replacement / exchange examples:
See page 170 for a solubility chart
PRECIPITATION of silver(I)
chloride drives this reaction!
In exchange reactions, transition metal
ions DO NOT change charge!
The FORMATION OF WATER
drives this reaction. It's a
NEUTRALIZATION. Detect by
evolution of HEAT
NO REACTION
NO REACTION occurs There is no DRIVING FORCE, since
both sodium chloride and potassium nitrate are both
soluble ionic compounds, They exist in water as
free ions (just like the original compounds do)
CARBONIC ACID decomposes
under these conditions to
form WATER and CARBON
DIOXIDE GAS (which escapes
as bubbles!)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5