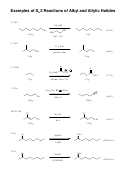
Example Of A Reactions That Forms Carbonic Acid, Then Gas: The "Baking Soda Volcano" Page 4
ADVERTISEMENT
124
SINGLE REPLACEMENT REACTIONS
One element, usually a metal, replaces another element in
a compound. This forms a new compound and leaves behind
a new free element!
example:
Silver gains electrons, goes
Copper loses electrons, goes
from +1 charge to 0 charge!
from 0 charge to +2 charge!
... but just because you combine an element and a compound doesn't mean that a reaction will
occur. Some combinations react, some don't!
- Whether a reaction occurs depends on how easily the replacing and replaced elements lose
electrons. An atom that loses electrons more easily will end up in IONIC form (in other words,
in the compound). An atom that loses electrons less easily will end up as a free element.
- We say that an atom that loses elecrons more easily that another is MORE ACTIVE than the
other element. But how would you get information about ACTIVITY?
A single replacement reaction is an example of a reaction where ELECTRON TRANSFER
is a driving force. Electron transfer reactions are generally called OXIDATION-REDUCTION
reactions.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5