Chemistry Worksheet With Answers - Cem 351 2nd Exam/version A Page 3
ADVERTISEMENT
2. (20 pts) Strong base treatment of 2-bromocyclopentanol can form a bicyclic ether, commonly
known as cyclopentene oxide, via an intramolecular S
2 reaction as shown. The base first
N
deprotonates the –OH to form an alkoxide, which then displaces the bromide as shown.
Base:
O
Br
H–O
Br
O
Br
cyclopentene
2-bromocyclopentanol
oxide
(a) (6 pts) Would cis- or trans-2-bromocyclopentanol be better for this reaction? Please
circle your answer and draw the compound in the box provided
Cis
Trans
HO
Br
(b) (2 pts) If the C-OH carbon has the (S) configuration, what is the configuration at the
C-Br carbon in your answer to (a)? Please circle your answer.
(R)
(S)
(c) (4 pts) Provide a name and formula for the hydrocarbon (shown) you would have if
the O atom in cyclopentene oxide were replaced by a CH
fragment.
2
Bicyclo[3.1.0]hexane
Name:
C
H
Formula:
6
10
(d) (4 pts) Cyclopentene oxide (an epoxide) can undergo S
2 attack by an external
N
–
nucleophile to displace O
, essentially the reverse of the process in (a). Draw the
–
product(s), including stereochemistry, that you would expect from attack of CH
S
.
3
HO
SCH
H
CS
OH
3
3
(e) (4 pts) Would you expect cyclopentene oxide to show any optical rotation? How
about your product mixture in (d)? Explain briefly.
Optical Rotation?
Meso (note the internal mirror plane)
Cyclopentene oxide: Yes
No
Product(s) in (d):
Yes
No
Racemic 50:50 mix of enantiomers
3
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4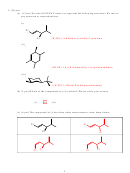 5
5 6
6








