Chemistry Worksheet With Answers - Cem 351 2nd Exam/version A Page 6
ADVERTISEMENT
6. (20 pts) In your environmentally conscientious search for cleaner-burning fuels, you consider
the following S
2 synthesis of the gasoline additive MTBE (methyl t-butyl ether):
N
-I ‡ (CH
–
+
+
–
(CH
)
C-O
K
+ CH
)
C-O-CH
+ K
+ I
(run in (CH
)
COH solvent)
3
3
3
3
3
3
3
3
(a) (4 pts) Identify the nucleophilic reactant in this process and circle it
–
+
(CH
)
C-O
K
CH
-I
3
3
3
(b) (4 pts) Draw curved arrows to show the flow of electrons in the reaction (redrawn below).
–
+
+
–
-I ‡ (CH
(CH
)
C-O
K
+ CH
)
C-O-CH
+ K
+ I
3
3
3
3
3
3
Igor, your new lab technician, attempts to repeat the reaction but confuses the reagents and
puts the following reaction together:
C-I ‡ ? (attempted in THF solvent)
–
+
CH
-O
K
+ (CH
)
3
3
3
At room temperature, not much happens, so Igor attempts to push the reaction by heating it.
Instead of getting his MTBE, he isolates a strong-smelling gas, which upon analysis proves
to have the formula C
H
.
4
8
(c) (6 pts) Why didn't Igor's experiment work to make MTBE via the S
2 reaction? Explain.
N
The S
2 reaction doesn’t work on a tertiary halide like t-butyl iodide because
N
approach from the back side of the C-I bond is blocked by the three methyl
groups. So elimination takes over when the reaction is pushed.
(d) (6 pts) What was the C
H
hydrocarbon that he did make instead (please draw it), and
4
8
what kind of reaction was responsible?
Igor made isobutylene (2-methylpropene) via an E2 reaction.
E1 would not work in the polar aprotic THF solvent; separation
of the ions is too difficult.
isobutylene
6
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4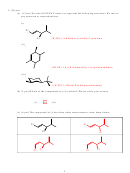 5
5 6
6








