Chemistry Worksheet With Answer Key - Chem 10113, Quiz 1b
ADVERTISEMENT
CHEM 10113, Exam 4
Name_____________________
November 30, 2011
(please print)
All equations must be balanced and show phases for full credit. Significant figures count, and
box your answers!
1. (10 points) Consider the phase diagram for iodine shown here and answer each of the
following questions.
184.4 C
a. What is the normal boiling point for iodine?
113.6 C
b. What is mp for iodine at 1 atm?
solid
c. What state is present at rt and normal atmospheric pressure?
gas
d. What state is present at 186 °C and 1.0 atm?
e. If a dynamic equilibrium is established between the solid and liquid phases, name
a change you can make to increase the amount of liquid.
Increase the temperature
2. (16 points) SHOW ALL WORK. Which of the following molecules are polar?
Polar
IF
5
BH
Nonpolar
3
1
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1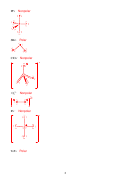 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9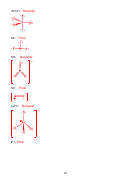 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17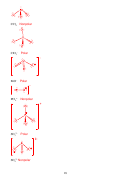 18
18 19
19 20
20 21
21 22
22 23
23 24
24








