Solubility Rules Chart
ADVERTISEMENT
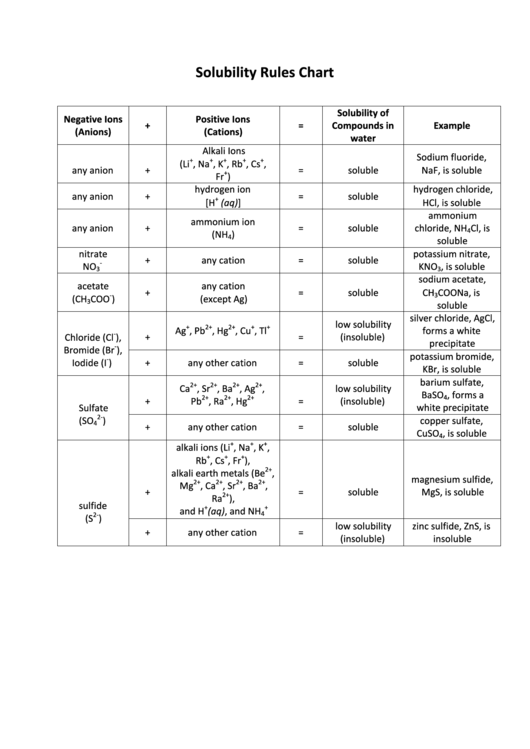
Solubility Rules Chart
Solubility of
Negative Ions
Positive Ions
+
=
Compounds in
Example
(Anions)
(Cations)
water
Alkali Ions
Sodium fluoride,
+
+
+
+
+
(Li
, Na
, K
, Rb
, Cs
,
any anion
+
=
soluble
NaF, is soluble
+
Fr
)
hydrogen ion
hydrogen chloride,
any anion
+
=
soluble
+
[H
(aq)]
HCl, is soluble
ammonium
ammonium ion
any anion
+
=
soluble
chloride, NH
Cl, is
4
(NH
)
4
soluble
nitrate
potassium nitrate,
+
any cation
=
soluble
-
NO
KNO
, is soluble
3
3
sodium acetate,
acetate
any cation
+
=
soluble
CH
COONa, is
-
3
(CH
COO
)
(except Ag)
3
soluble
silver chloride, AgCl,
low solubility
+
2+
2+
+
+
Ag
, Pb
, Hg
, Cu
, Tl
forms a white
-
+
=
(insoluble)
Chloride (Cl
),
precipitate
-
Bromide (Br
),
potassium bromide,
-
Iodide (I
)
+
any other cation
=
soluble
KBr, is soluble
barium sulfate,
2+
2+
2+
2+
Ca
, Sr
, Ba
, Ag
,
low solubility
BaSO
, forms a
2+
2+
2+
4
+
Pb
, Ra
, Hg
=
(insoluble)
white precipitate
Sulfate
2-
(SO
)
copper sulfate,
4
+
any other cation
=
soluble
CuSO
, is soluble
4
+
+
+
alkali ions (Li
, Na
, K
,
+
+
+
Rb
, Cs
, Fr
),
2+
alkali earth metals (Be
,
magnesium sulfide,
2+
2+
2+
2+
Mg
, Ca
, Sr
, Ba
,
+
=
soluble
MgS, is soluble
2+
Ra
),
sulfide
+
+
and H
(aq), and NH
4
2-
(S
)
low solubility
zinc sulfide, ZnS, is
+
any other cation
=
(insoluble)
insoluble
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2