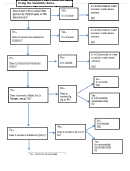
Solubility Rules
ADVERTISEMENT
Solubility Rules
Apply the Rules in Order
+
+
+
+
1. All Li
, Na
, K
, and NH
compounds are soluble
4
-
-
-
2. All NO
, ClO
, CH
CO
compounds are soluble
3
4
3
2
+
2+
4+
2+
3. All Ag
, Pb
, Pb
, and Hg
compounds are insoluble
2
-
-
-
-
4. All F
, Cl
, Br
, and I
compounds are soluble
2-
2-
3-
2-
-
5. All S
, O
, PO
, CO
, OH
are insoluble
4
3
2-
2+
2+
6. All SO
compounds except Ca
and Ba
are soluble
4
7. Any compound not found on this chart is insoluble
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1