Molecular Orbital Practice Problems Answers - Western Connecticut
ADVERTISEMENT
Western Connecticut State University
Molecular Orbital Theory
General Chemistry I CHE 110
These problems are for practice in drawing your molecular orbital diagrams, molecular electron
configurations and determining bond order.
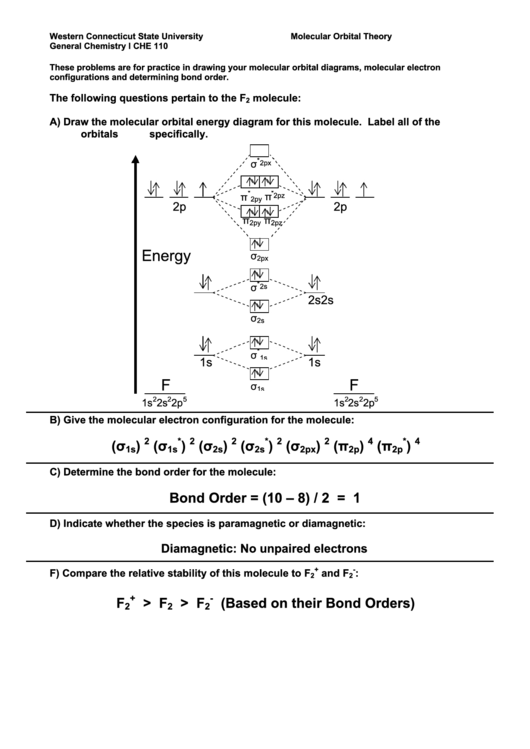
The following questions pertain to the F
molecule:
2
A)
Draw the molecular orbital energy diagram for this molecule. Label all of the
orbitals specifically.
*
σ
2px
*
*
π
π
2py
2pz
2p
2p
π
π
2py
2pz
Energy
σ
2px
*
σ
2s
2s
2s
σ
2s
*
σ
1s
1s
1s
F
F
σ
1s
2
2
5
2
2
5
1s
2s
2p
1s
2s
2p
B)
Give the molecular electron configuration for the molecule:
2
*
2
2
*
2
2
4
*
4
(σ
)
(σ
)
(σ
)
(σ
)
(σ
)
(π
)
(π
)
1s
1s
2s
2s
2px
2p
2p
C)
Determine the bond order for the molecule:
Bond Order = (10 – 8) / 2 = 1
D)
Indicate whether the species is paramagnetic or diamagnetic:
Diamagnetic: No unpaired electrons
+
-
F)
Compare the relative stability of this molecule to F
and F
:
2
2
+
-
F
> F
> F
(Based on their Bond Orders)
2
2
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3








