Chemistry Questions Molecular Polarity Page 2
ADVERTISEMENT
Lone pair electrons double bonds, and triple bonds are also areas of molecules that are slightly negative
because of the increased charge density.
2.
Charge density triple bond > charge density double bond> charge density of single bond
Why does increasing the number of pi bonds add to charge density?
The distribution of negative and positive charges in the molecule determines is the molecule itself is polar. The
greater the difference between the center of positive charges and the center of negative charges the more polar
the molecule is. The distance from the center of positive charges to the center of negative charges is a vector
quantity called dipole moment.
3.
Build models and draw sketches of CH
, CH
Cl, CH
Cl
, CHCl
, and CCl
4
3
2
2
3
4
Is the carbon-hydrogen bond polar?
Is the carbon-chlorine bond polar? Which atom is negative and which is positive?
The center of negative charge is in the middle of the chlorines and the center of positive charges is the carbon.
Which of the CH
Cl, CH
Cl
, CHCl
, and CCl
molecules has the biggest distance between the center of
3
2
2
3
4
positive and negative charges?
Why is the center of negative and positive charges the same in CCl
?
4
4.
Draw a sketch of BBr
3
Is the boron-bromine bond polar or nonpolar?
Locate the center of positive and negative charge in the BBr
molecule. Why is the molecule nonpolar?
3
In general molecular polarity can be summed up as follows:
Negative areas of a molecule are caused by more the electronegative atom of polar bonds, with the strength of
the negative charge increasing with higher percentage ionic character. Lone pairs and pi bonds produce weaker
areas of negative charge.
If a molecule has no polar bonds, lone pairs, or pi bonds it cannot be polar.
If a molecule has negative charges distributes symmetrically it is not polar.
If polar bonds are on the opposite end of a molecule from nonpolar bonds the molecules will be polar because
the centers of positive and negative charge will be far apart.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Miscellaneous
 1
1 2
2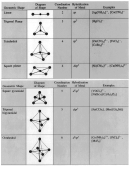 3
3 4
4