Chemistry Notes - Measurement & Calculations Involving The Mole Page 2
ADVERTISEMENT
Chemistry Notes
Chapters 5,6 - Measurement & Calculations Involving the Mole
Section 6.1-6.3 - Moles, Molar Mass, Percent Composition and Empirical Formula
Avogadro = Italian scientist and mathematician who calculated the number of particles that make
up a mole of gas must be proportional to its volume
Mole = a unit of measurement in chemistry to represent:
1. (Quantity) Avogadro's Number of particles = 6.022 x 10ˆ23
2. (Mass) Equivalent to 12.0 grams of carbon-12
Molar mass = mass of one mole of a substance; equivalent to the atomic mass unit listed on the
periodic table
Formula mass = sum of all of the atomic mass units of all of the atoms in a chemical compound
Steps -
1. Count up the number of atoms (using the subscripts)
2. Multiply each element by its molar mass
3. Add up all of the molar masses to find the formula mass
Percent composition = percentage by mass of each element in a chemical compound
Steps -
1. Calculate the formula mass (total mass of compound)
2. Divide the individual mass of each element by the total mass of the compound
3. Multiply by 100 to find the percentage
Empirical formula = shows the smallest whole-number ratio of elements in a chemical compound
Steps -
1. Convert the given mass to moles
2. Divide by the smallest number of moles to find the smallest whole-number mole ratio
3. Use the mole ratio numbers as subscripts for the empirical formula
Molecular formula = any whole-number multiple of the empirical formula
Steps -
1. Calculate the empirical formula mass
2. Divide the total molecular formula mass by the empirical formula mass to find the number of
molecules
3. Use the number of molecules as the subscripts for the molecular formula
Mrs. Drurey
- 2 of 3 -
Newton South High School
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2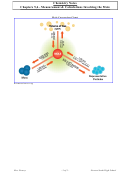 3
3








