Reading The Solubility Chart
ADVERTISEMENT
Reading a Solubility Chart
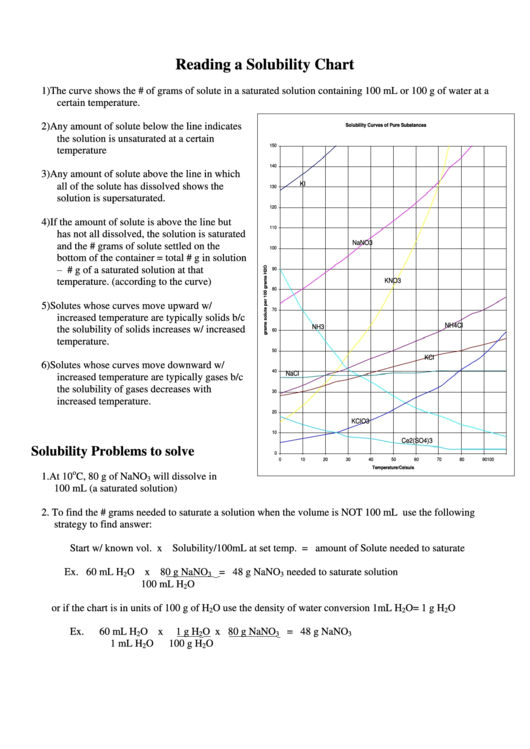
1) The curve shows the # of grams of solute in a saturated solution containing 100 mL or 100 g of water at a
certain temperature.
2) Any amount of solute below the line indicates
Solubility Curves of Pure Substances
the solution is unsaturated at a certain
150
temperature
140
3) Any amount of solute above the line in which
KI
all of the solute has dissolved shows the
130
solution is supersaturated.
120
4) If the amount of solute is above the line but
110
has not all dissolved, the solution is saturated
NaNO3
and the # grams of solute settled on the
100
bottom of the container = total # g in solution
– # g of a saturated solution at that
90
temperature. (according to the curve)
KNO3
80
5) Solutes whose curves move upward w/
70
increased temperature are typically solids b/c
NH4Cl
NH3
the solubility of solids increases w/ increased
60
temperature.
50
KCl
6) Solutes whose curves move downward w/
40
NaCl
increased temperature are typically gases b/c
the solubility of gases decreases with
30
increased temperature.
20
KClO3
10
Ce2(SO4)3
Solubility Problems to solve
0
0
10
20
30
40
50
60
70
80
90
100
Temperature/Celsuis
o
1. At 10
C, 80 g of NaNO
will dissolve in
3
100 mL (a saturated solution)
2. To find the # grams needed to saturate a solution when the volume is NOT 100 mL use the following
strategy to find answer:
Start w/ known vol. x Solubility/100mL at set temp. = amount of Solute needed to saturate
Ex. 60 mL H
O x 80 g NaNO
= 48 g NaNO
needed to saturate solution
2
3
3
100 mL H
O
2
or if the chart is in units of 100 g of H
O use the density of water conversion 1mL H
O= 1 g H
O
2
2
2
Ex.
60 mL H
O x
1 g H
O x 80 g NaNO
= 48 g NaNO
2
2
3
3
1 mL H
O
100 g H
O
2
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2