Common And Polyatomic Ions Chart
ADVERTISEMENT
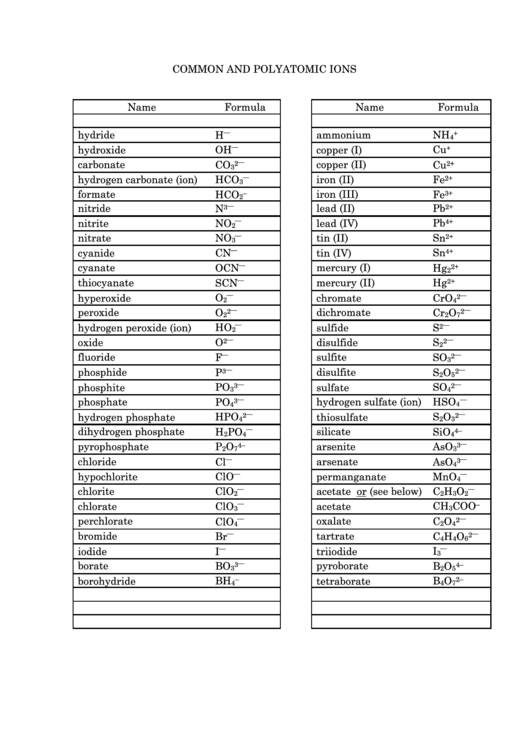
COMMON AND POLYATOMIC IONS
Name
Formula
Name
Formula
hydride
H
⎯
ammonium
NH
+
4
hydroxide
OH
⎯
copper (I)
Cu
+
carbonate
CO
⎯
copper (II)
Cu
2
2+
3
hydrogen carbonate (ion)
⎯
iron (II)
Fe
HCO
2+
3
formate
HCO
iron (III)
Fe
–
3+
2
nitride
N
⎯
lead (II)
Pb
2+
3
nitrite
NO
⎯
lead (IV)
Pb
4+
2
nitrate
NO
⎯
tin (II)
Sn
2+
3
cyanide
CN
⎯
tin (IV)
Sn
4+
cyanate
⎯
mercury (I)
Hg
OCN
2+
2
thiocyanate
⎯
mercury (II)
Hg
SCN
2+
hyperoxide
O
⎯
chromate
CrO
⎯
2
2
4
peroxide
⎯
dichromate
⎯
O
Cr
O
2
2
2
2
7
hydrogen peroxide (ion)
HO
⎯
sulfide
S
⎯
2
2
oxide
O
⎯
disulfide
S
⎯
2
2
2
fluoride
F
⎯
sulfite
SO
⎯
2
3
phosphide
⎯
disulfite
⎯
P
S
O
3
2
2
5
phosphite
⎯
sulfate
⎯
PO
SO
3
2
3
4
phosphate
PO
⎯
hydrogen sulfate (ion)
HSO
⎯
3
4
4
hydrogen phosphate
⎯
thiosulfate
⎯
HPO
S
O
2
2
4
2
3
dihydrogen phosphate
⎯
silicate
SiO
H
PO
4–
4
2
4
pyrophosphate
P
O
arsenite
AsO
⎯
4–
3
2
7
3
chloride
Cl
⎯
arsenate
AsO
⎯
3
4
hypochlorite
ClO
⎯
permanganate
MnO
⎯
4
chlorite
ClO
⎯
acetate or (see below) C
H
O
⎯
2
2
3
2
chlorate
ClO
⎯
acetate
CH
COO
–
3
3
perchlorate
⎯
oxalate
⎯
ClO
C
O
2
4
2
4
bromide
⎯
tartrate
⎯
Br
C
H
O
2
4
4
6
iodide
I
⎯
triiodide
I
⎯
3
borate
BO
⎯
pyroborate
B
O
4–
3
3
2
5
borohydride
BH
tetraborate
B
O
–
2–
4
4
7
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








