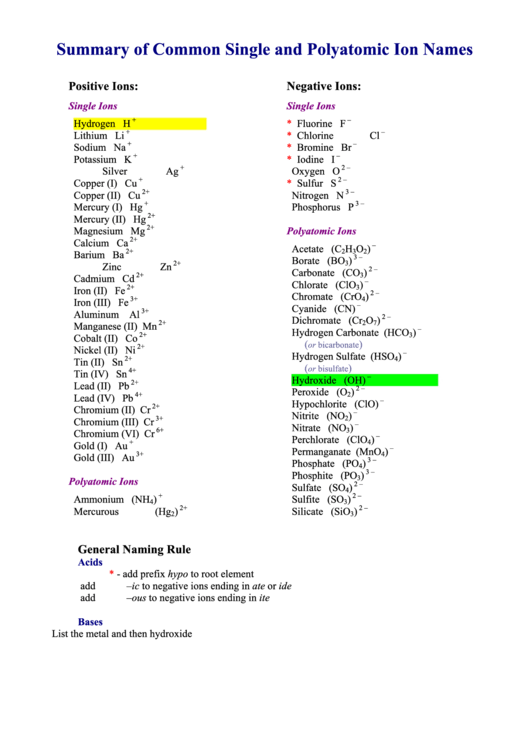
Common Single And Polyatomic Ion Names Chart
ADVERTISEMENT
Summary of Common Single and Polyatomic Ion Names
Positive Ions:
Negative Ions:
Single Ions
Single Ions
+
–
Hydrogen
H
*
Fluorine
F
+
–
Lithium
Li
*
Chlorine
Cl
+
–
Sodium
Na
*
Bromine
Br
+
–
Potassium
K
*
Iodine
I
+
2 –
Silver
Ag
Oxygen
O
+
2 –
Copper (I)
Cu
*
Sulfur
S
2+
3 –
Copper (II)
Cu
Nitrogen
N
+
3 –
Mercury (I)
Hg
Phosphorus
P
2+
Mercury (II)
Hg
2+
Magnesium
Mg
Polyatomic Ions
2+
Calcium
Ca
–
Acetate
(C
H
O
)
2+
2
3
2
Barium
Ba
3 –
Borate
(BO
)
2+
3
Zinc
Zn
2 –
Carbonate
(CO
)
2+
3
Cadmium
Cd
–
Chlorate
(ClO
)
2+
3
Iron (II)
Fe
2 –
Chromate
(CrO
)
3+
4
Iron (III)
Fe
–
Cyanide
(CN)
3+
Aluminum
Al
2 –
Dichromate
(Cr
O
)
2+
2
7
Manganese (II)
Mn
–
Hydrogen Carbonate
(HCO
)
2+
3
Cobalt (II)
Co
(
)
or bicarbonate
2+
Nickel (II)
Ni
–
Hydrogen Sulfate
(HSO
)
2+
4
Tin (II)
Sn
(
)
or bisulfate
4+
Tin (IV)
Sn
–
Hydroxide
(OH)
2+
Lead (II)
Pb
2 –
Peroxide
(O
)
4+
2
Lead (IV)
Pb
–
Hypochlorite
(ClO)
2+
Chromium (II)
Cr
–
Nitrite
(NO
)
3+
2
Chromium (III)
Cr
–
Nitrate
(NO
)
6+
3
Chromium (VI)
Cr
–
Perchlorate
(ClO
)
+
4
Gold (I)
Au
–
Permanganate
(MnO
)
3+
4
Gold (III)
Au
3 –
Phosphate
(PO
)
4
3 –
Phosphite
(PO
)
3
Polyatomic Ions
2 –
Sulfate
(SO
)
4
+
2 –
Ammonium
(NH
)
Sulfite
(SO
)
4
3
2 –
2+
Mercurous
(Hg
)
Silicate
(SiO
)
2
3
General Naming Rule
Acids
*
- add prefix hypo to root element
add –ic to negative ions ending in ate or ide
add –ous to negative ions ending in ite
Bases
List the metal and then hydroxide
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








