Naming Compounds Flow Chart
ADVERTISEMENT
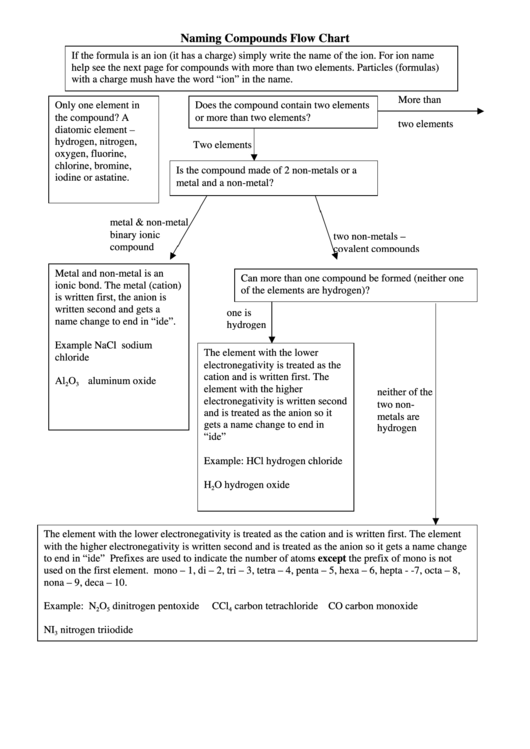
Naming Compounds Flow Chart
If the formula is an ion (it has a charge) simply write the name of the ion. For ion name
help see the next page for compounds with more than two elements. Particles (formulas)
with a charge mush have the word “ion” in the name.
More than
Only one element in
Does the compound contain two elements
the compound? A
or more than two elements?
two elements
diatomic element –
hydrogen, nitrogen,
Two elements
oxygen, fluorine,
chlorine, bromine,
Is the compound made of 2 non-metals or a
iodine or astatine.
metal and a non-metal?
metal & non-metal
binary ionic
two non-metals –
compound
covalent compounds
Metal and non-metal is an
Can more than one compound be formed (neither one
ionic bond. The metal (cation)
of the elements are hydrogen)?
is written first, the anion is
written second and gets a
one is
name change to end in “ide”.
hydrogen
Example NaCl sodium
The element with the lower
chloride
electronegativity is treated as the
cation and is written first. The
Al
O
aluminum oxide
2
3
element with the higher
neither of the
electronegativity is written second
two non-
and is treated as the anion so it
metals are
gets a name change to end in
hydrogen
“ide”
Example: HCl hydrogen chloride
H
O hydrogen oxide
2
The element with the lower electronegativity is treated as the cation and is written first. The element
with the higher electronegativity is written second and is treated as the anion so it gets a name change
to end in “ide” Prefixes are used to indicate the number of atoms except the prefix of mono is not
used on the first element. mono – 1, di – 2, tri – 3, tetra – 4, penta – 5, hexa – 6, hepta - -7, octa – 8,
nona – 9, deca – 10.
Example: N
O
dinitrogen pentoxide
CCl
carbon tetrachloride CO carbon monoxide
2
5
4
NI
nitrogen triiodide
3
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Business
 1
1 2
2