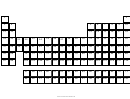
Circular Periodic Table Page 2
ADVERTISEMENT
Circular Periodic Table
Sl.No.
Name
of
Period
Contain
Number of
Atomic
Description
period
Number
shell (S)
element
Number
01
sp
1
s, p
2
01 to 02
H
to He
02
p
2
s, p,
8
03 to 10
Li
to Ne
1
03
p
3
s, p,
8
11 to 18
Na
to Ar
2
04
d
4
s, p, d
18
19 to 36
K
to Kr
1
05
d
5
s, p, d
18
37 to 54
Rb
to Xe
2
06
f
6
s, p, d, f
32
55 to 86
Cs
to Rn
1
07
f
7
s, p, d, f
32
87 to 118
Fr
to Uuo
2
08
g
8
s, p, d, f,
50
119 to 168
Uue to Uho
1
g
to ….
09
g
9
s, p, d, f,
50
169 to 218
Une
2
g
219 to….
………
10
h
10
s, p, d, f,
.
1
h
…..
…….
…….
………
…….
……..
…………
Fig- 1
Rule:“The element exist in a circular Periodic table in order to your increasing positive charged
energy level.” The positive charged energies consider by the scientist as the atomic number of an element. So
the element exist in a periodic table in order to your increasing atomic number. Here it is also a noting points
that in an element the positive charged energy level is equal to negative charged energy level. Therefore the
element electrically neutral.
Border Line
Fig- 2
Note:
There is no theoretical changing in long form of periodic table, extended & dynamical form of periodic
table etc.The properties of groups, periods & blocks are just same. Here produce graphical changing with
addition of some other properties, concept, formule, potion for new elements etc.
Concept for status of an element:- If E is an element then,
{ E }
(Z,B,G,H,P)
35 | P a g e
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4