Circular Periodic Table Page 3
ADVERTISEMENT
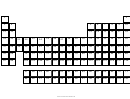
Circular Periodic Table
Show that E is an element. and exist in B-block, G group, H head group and P period contains atomic number Z.
Such that consider an element contains atomic number 88, that is Ra (radium) then the figure-
{Ra}
(88,2,1,f2)
nd
shows that Ra is a element exist in S-block, 2
group ,first head group & f
period contains atomic number 88.
2
Group :-Theoritcally there consider 8 group 1, 2, 3, 4, 5, 6, 7, 8 as a head group, given below-
st
1.
Defined as 1
head group
nd
2.
Defined as 2
head group
rd
3.
Defined as 3
head group
th
4.
Defined as 4
head group
th
5.
Defined as 5
head group
th
6.
Defined as 6
head group
th
7.
Defined as 7
head group
th
8.
Defined as 8
head group
st
Hydrogen & Helium defined as group supperitendant and Hydrogen exist under group 1
, Helium under group
th
8
.
The group contains elements :-
st
period – one element Li atomic number 03.
1
head group in- p
1
period – one element Na atomic number 11.
p
2
period – two elements atomic number 19 to 20.
d
1
period – two elements atomic number 37 to 38.
d
2
period – four elements atomic number 55 to 58.
f
1
period – four elements atomic number 87 to 90.
f
2
period – six elements atomic number 119 to 124.
g
1
period – six elements atomic number 169 to 174.
g
2
nd
period – one element Be atomic number 4.
2
head group in- p
1
period – one element Ti atomic number 12.
p
2
period – three elements atomic number 21 to 23.
d
1
period – three elements atomic number 39 to 41.
d
2
period – four elements atomic number 59 to 62.
f
1
period – four elements atomic number 91 to 94.
f
2
period – seven elements atomic number 125 to 131.
g
1
period – seven elements atomic number 175 to 181.
g
2
rd
period – one element B atomic number 5.
3
head group in-p
1
period – one element Be atomic number 13.
p
2
period – two elements atomic number 24 to 25.
d
1
period – two elements atomic number 42 to 43.
d
2
period – four elements atomic number 63 to 66.
f
1
period – four elements atomic number 95 to 98.
f
2
period – seven elements atomic number 132 to 137.
g
1
period – seven elements atomic number 182 to 187.
g
2
th
period – one element C atomic number 6.
4
head group in-p
1
period – one element Si atomic number 14.
p
2
period – two elements atomic number 26 to 27.
d
1
period – two elements atomic number 44 to 45.
d
2
period – four elements atomic number 67 to 70.
f
1
period – four elements atomic number 99 to 102.
f
2
period – six elements atomic number 138 to 143.
g
1
period – six elements atomic number 188 to 193.
g
2
th
period – one element N atomic number 7.
5
head group in- p
1
period – one element S atomic number 15.
p
2
period – two elements atomic number 28 to 29.
d
1
period – two elements atomic number 46 to 47.
d
2
period – four elements atomic number 71 to 74.
f
1
period – four elements atomic number 103 to 106.
f
2
period – six elements atomic number 144 to 150.
g
1
period – six elements atomic number 194 to 200.
g
2
th
period – one element O atomic number 8.
6
head group in- p
1
period – one element P atomic number 16.
p
2
period – three elements atomic number 30 to 32.
d
1
36 | P a g e
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4