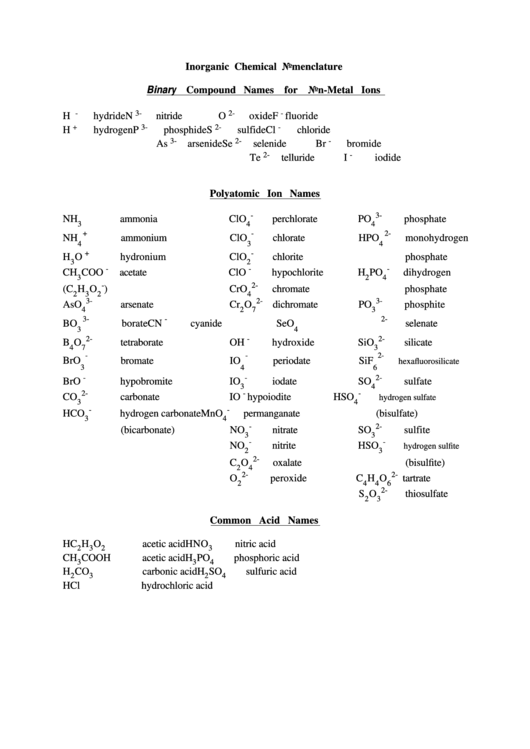
Inorganic Chemical Nomenclature Chart
ADVERTISEMENT
Inorganic Chemical Nomenclature
Binary Compound Names for Non-Metal Ions
-
3-
2-
-
H
hydride
N
nitride
O
oxide
F
fluoride
+
3-
2-
-
H
hydrogen
P
phosphide
S
sulfide
Cl
chloride
3-
2-
-
As
arsenide
Se
selenide
Br
bromide
2-
-
Te
telluride
I
iodide
Polyatomic Ion Names
-
3-
NH
ammonia
ClO
perchlorate
PO
phosphate
3
4
4
+
-
2-
NH
ammonium
ClO
chlorate
HPO
monohydrogen
4
3
4
+
-
H
O
hydronium
ClO
chlorite
phosphate
3
2
-
-
-
CH
COO
acetate
ClO
hypochlorite
H
PO
dihydrogen
3
2
4
-
2-
(C
H
O
)
CrO
chromate
phosphate
2
3
2
4
3-
2-
3-
AsO
arsenate
Cr
O
dichromate
PO
phosphite
4
2
7
3
3-
-
2-
BO
borate
CN
cyanide
SeO
selenate
3
4
2-
-
2-
B
O
tetraborate
OH
hydroxide
SiO
silicate
4
7
3
-
-
2-
BrO
bromate
IO
periodate
SiF
hexafluorosilicate
3
4
6
-
-
2-
BrO
hypobromite
IO
iodate
SO
sulfate
3
4
2-
-
-
CO
carbonate
IO
hypoiodite
HSO
hydrogen sulfate
3
4
-
-
HCO
hydrogen carbonate
MnO
permanganate
(bisulfate)
3
4
-
2-
(bicarbonate)
NO
nitrate
SO
sulfite
3
3
-
-
NO
nitrite
HSO
hydrogen sulfite
2
3
2-
C
O
oxalate
(bisulfite)
2
4
2-
2-
O
peroxide
C
H
O
tartrate
2
4
4
6
2-
S
O
thiosulfate
2
3
Common Acid Names
HC
H
O
acetic acid
HNO
nitric acid
2
3
2
3
CH
COOH
acetic acid
H
PO
phosphoric acid
3
3
4
H
CO
carbonic acid
H
SO
sulfuric acid
2
3
2
4
HCl
hydrochloric acid
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








