Chemistry Worksheets
ADVERTISEMENT
CHEMISTRY 253
Spring, 2015 - Dixon
Homework Set 1.3
Solutions – Non-Collected Problems
Problems: 3-2, 3-6, 3-7
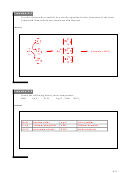
3-2. By adding up the three reactions, show that the net result of the photochemical
decomposition of NO
, the formation of ozone from atomic oxygen, and the above reaction
2
constitute no overall reaction, i.e., a null process.
ν
→ NO + O
1)
NO
+ h
2
+ M → O
2)
O + O
+ M
2
3
→ NO
3)
NO + O
+ O
3
2
2
all products and reactants can be cancelled to give a net null process
3-6
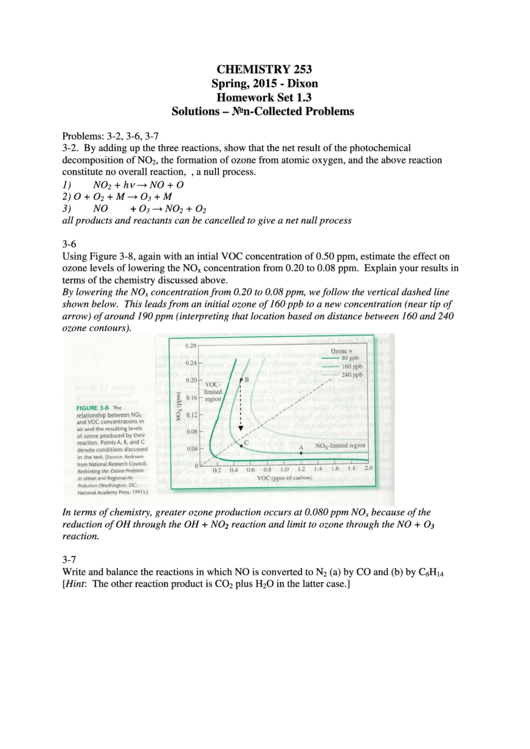
Using Figure 3-8, again with an intial VOC concentration of 0.50 ppm, estimate the effect on
ozone levels of lowering the NO
concentration from 0.20 to 0.08 ppm. Explain your results in
x
terms of the chemistry discussed above.
By lowering the NO
concentration from 0.20 to 0.08 ppm, we follow the vertical dashed line
x
shown below. This leads from an initial ozone of 160 ppb to a new concentration (near tip of
arrow) of around 190 ppm (interpreting that location based on distance between 160 and 240
ozone contours).
In terms of chemistry, greater ozone production occurs at 0.080 ppm NO
because of the
x
reduction of OH through the OH + NO
reaction and limit to ozone through the NO + O
2
3
reaction.
3-7
Write and balance the reactions in which NO is converted to N
(a) by CO and (b) by C
H
2
6
14
[Hint: The other reaction product is CO
plus H
O in the latter case.]
2
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3