Qualitative Analysis Page 2
ADVERTISEMENT
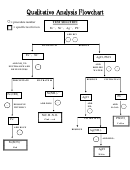
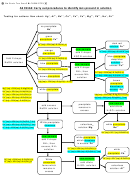
7.
The approximate pH of a solution is determined by removing a drop of the solution with
a stirring rod and touching it to a piece of universal indicator paper.
8.
A 100 or 150 ml beaker of boiling distilled water is used to heat solutions in test tubes.
9.
To perform a precipitation, add the indicated reagent to your test tube and mix
thoroughly.
Heat the solution if so directed (this sometimes help to form larger
particles). When the precipitation appears to be complete, centrifuge the sample. Before
removing the supernatant liquid, add a drop of the precipitating reagent.
If the
precipitation is incomplete, more precipitate will form in the liquid. If this is the case,
add a few more drops, centrifuge, and test again.
10.
To centrifuge a sample, place the test tube in one opening, and counter balance with
another test tube with a similar volume of solution. Run for about 30 seconds, but this
may vary with the nature of the precipitate. Cooperate with the centrifuge as we have
only three.
The supernatant liquid is usually removed (to another test tube) by
decantation. A capillary pipet can also be used. The precipitate must be washed, usually
with DW. Add a few milliliters, shake, centrifuge, and discard supernatant liquid.
Repeat.
11.
Keep careful records of colors, textures, odors, reactions, etc in your notebook.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Business
 1
1 2
2 3
3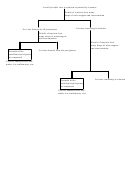 4
4