Qualitative Analysis Page 3
ADVERTISEMENT
GROUP 1 CATIONS
2+
2+
+
Pb
, Hg
, Ag
2
1.
Add 10 drops of 0.1 M solutions of the three ions in three separate test tubes. Add 4
drops of 6 M HCl to each. As always, observe the results. Keep in mind that you will
write balanced equations, usually the net ionic, for any observed reactions throughout
these procedures. The equations do not have to be recorded in your notebook as you go,
but it is a good idea to do so to keep track of the reactions.
2.
Add 14 drops of DW to any washed precipitates (in step #1) and heat in your water bath.
3.
To any solutions formed (#2), add 2 drops 6 M acetic acid and 3 drops 1 M K
CrO
.
2
4
4.
To any precipitates (#1) which do not dissolve in hot water, add 10 drops of 6 M NH
.
3
5.
Acidify (to test paper) any solutions formed (#4) with 20 drops 6 M HNO
. Make certain
3
the solution is acidic.
The reactions in #3, #4, and #5 are confirmatory tests for that specific ion when it is the only ion
possible in solution.
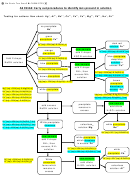
Develop a procedure for separating, and confirming the presence of the three ions in a prepared
mixture. This is usually written in the form of a flow chart. Possible ions are shown at the top.
Reagents are shown on vertical lines. If a precipitate forms, the precipitate is separated, and
indicated on the left with a double vertical line. The supernatant liquid goes on the right with a
single line. Confirmatory tests are blocked. Prepare a mixture of the three ions (about 4
drops of each) and test your procedure.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Business
 1
1 2
2 3
3 4
4