Chemistry Worksheet Page 2
ADVERTISEMENT
10 Why are valence electrons important in how atoms react?
11. How many valence electrons do each of the following have?
a. I _____
b. B ____
c. Ca ____
d. He ____
e. Ar _____
12. Why are metals malleable?
13. Why are ionic compounds brittle?
14. Why do metals conduct electricity?
15. When can ionic compounds conduct electricity? When can’t they?
16. Why can’t we say “molecules” of NaCl?
17. What is a polar bond?
18. What is a polyatomic ion?
19. Use electron dot diagrams to show how the following form ionic bonds
a. K and N
b. Be and I
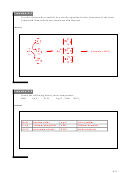
20. Use electron dot diagrams to show how the following form covalent bonds
a. F
O
b. NI
c. N
2
3
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2