Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution
ADVERTISEMENT
Carboxylic Acid Derivatives:
Nucleophilic Acyl Substitution
SUMMARY
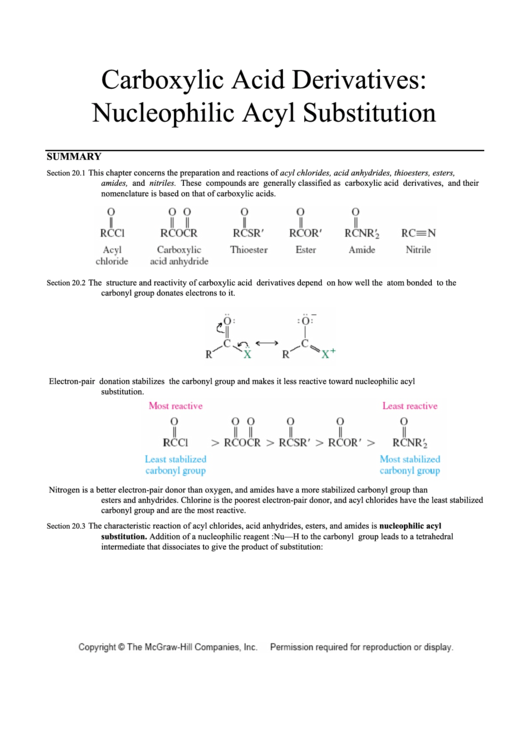
This chapter concerns the preparation and reactions of acyl chlorides, acid anhydrides, thioesters, esters,
Section 20.1
amides, and nitriles. These compounds are generally classified as carboxylic acid derivatives, and their
nomenclature is based on that of carboxylic acids.
The structure and reactivity of carboxylic acid derivatives depend on how well the atom bonded to the
Section 20.2
carbonyl group donates electrons to it.
Electron-pair donation stabilizes the carbonyl group and makes it less reactive toward nucleophilic acyl
substitution.
Nitrogen is a better electron-pair donor than oxygen, and amides have a more stabilized carbonyl group than
esters and anhydrides. Chlorine is the poorest electron-pair donor, and acyl chlorides have the least stabilized
carbonyl group and are the most reactive.
The characteristic reaction of acyl chlorides, acid anhydrides, esters, and amides is nucleophilic acyl
Section 20.3
substitution. Addition of a nucleophilic reagent :Nu—H to the carbonyl group leads to a tetrahedral
intermediate that dissociates to give the product of substitution:
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4








