Ions Worksheet
ADVERTISEMENT
Name: ______________________ Number:
Ions
Pd: _________ Date: _________________
How are ions made from neutral atoms?
Why?
You have learned that not all atoms of an element are the same. Variation in the number of neutrons results in
different isotopes of the element. In this activity we will explore another variation that can take place—the loss and gain of
electrons. The exchange of electrons between atoms is a very common way for chemical change to take place. We will see it
many times throughout the year.
Model 1
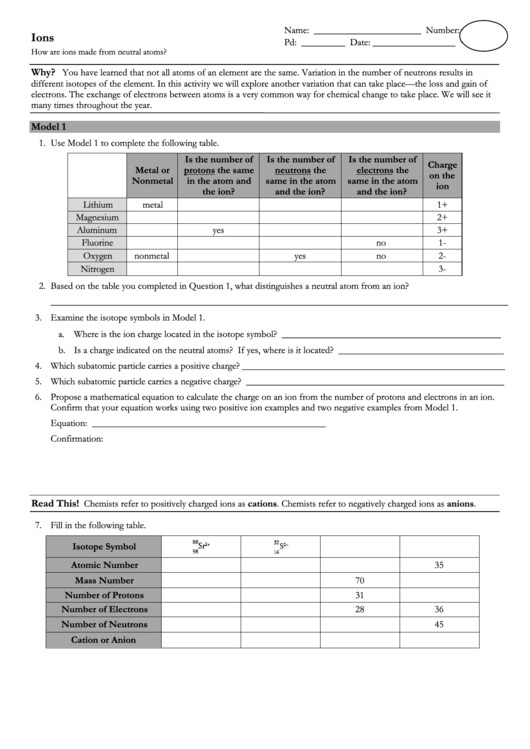
1. Use Model 1 to complete the following table.
Is the number of
Is the number of
Is the number of
Charge
Metal or
protons the same
neutrons the
electrons the
on the
Nonmetal
in the atom and
same in the atom
same in the atom
ion
the ion?
and the ion?
and the ion?
Lithium
metal
1+
Magnesium
2+
Aluminum
yes
3+
Fluorine
no
1-
Oxygen
nonmetal
yes
no
2-
Nitrogen
3-
2. Based on the table you completed in Question 1, what distinguishes a neutral atom from an ion?
______________________________________________________________________________________________
3. Examine the isotope symbols in Model 1.
a. Where is the ion charge located in the isotope symbol? _____________________________________________
b. Is a charge indicated on the neutral atoms? If yes, where is it located? __________________________________
4. Which subatomic particle carries a positive charge? ______________________________________________________
5. Which subatomic particle carries a negative charge? _____________________________________________________
6. Propose a mathematical equation to calculate the charge on an ion from the number of protons and electrons in an ion.
Confirm that your equation works using two positive ion examples and two negative examples from Model 1.
Equation: ________________________________________________
Confirmation:
Read This!
Chemists refer to positively charged ions as cations. Chemists refer to negatively charged ions as anions.
7. Fill in the following table.
Isotope Symbol
Atomic Number
35
Mass Number
70
Number of Protons
31
Number of Electrons
28
36
Number of Neutrons
45
Cation or Anion
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








