Problems For Bronsted-Lowry Acid-Base Chemistry Worksheet With Answers Page 2
ADVERTISEMENT
3. Fill in the reactants or products for the following acid-base reactions. Keep in mind
that in line-angle formulas hydrogens are not shown. When in doubt, write complete
Lewis structures. Abbreviations used: Ph=phenyl (a benzene ring attached to a carbon
chain)
HNO
+ H
O
3
2
F
+ H
O
3
O
+
OH
H
C
CH
3
3
PhCOO
+ HCl
H
CO
+ OH
2
3
+ H
O
2
NH
+ HC C
3
OH + H
4. Arrange the substances by order of acidity or basicity as indicated. You may use pKa
tables, periodic tables, or any other tools available to you (except for your cell phone).
H
S
H
Se
H
O
2
2
2
strongest acid
weakest acid
Br
F
I
Cl
weakest base
strongest base
CH
HI
PH
H
Se
4
3
2
strongest acid
weakest acid
F
OH
NH
CH
CH
CH
CH
2
3
2
2
2
strongest base
weakest basse
CH
CH
OH
CH
COOH
HF
Benzene
3
2
3
strongest acid
weakest acid
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4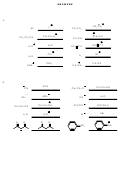 5
5 6
6 7
7 8
8








