Problems For Bronsted-Lowry Acid-Base Chemistry Worksheet With Answers Page 3
ADVERTISEMENT
5. Circle the side favored by equilibrium in the following acid-base reactions.
CN
+
NH
HCN
+
NH
3
2
O
O
+
+
O
OH
H
C
CH
H
C
CH
3
2
3
3
H
O +
H
O
+
NH
NH
2
3
2
3
H
CO
+ H
O
HCO
+
H
O
2
3
2
3
3
CH
CH
CH
CH
+
CH
CH
CH
CH
+
CH
CH
3
2
2
3
3
2
2
2
2
3
6. The conjugate acid of ammonia, NH
, is:
3
+
-
A) NH
B) NH
OH
C) NH
D) none of the above
4
2
2
7. Methanesulfonic acid, CH
SO
H, has a pK
of -7 while ethanol, CH
CH
OH, has a pK
of
a
a
3
3
3
2
15.9. Which is the stronger acid and what accounts for this large difference in relative
acidity?
8. Would you predict trifluoromethanesulfonic acid, CF
SO
H, to be a stronger or weaker
3
3
acid than methanesulfonic acid, CH
SO
H? Explain your reasoning.
3
3
O - , NH
- , and CH
COO - . Rank these ions in order of
9. Consider the species CH
3
2
3
increasing basicity, and explain your rationale.
of formic acid is 1.7 x 10 -4 . The pK
10. The K
of formic acid is __________.
a
a
A) 1.7
B) 10.3
C) 4.0
D) 3.8
E) -2.3
11. Provide the structure of the conjugate base of phenol (shown below) and all its
resonance forms.
OH
12. Rank the following in order of increasing acidity: CH
OH, HCl, NH
, and CH
.
3
3
4
O - , H
N - , H
13. Rank the following in order of increasing basicity: CH
O, and NH
.
3
2
2
3
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4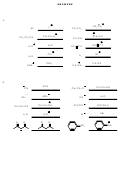 5
5 6
6 7
7 8
8








