Problems For Bronsted-Lowry Acid-Base Chemistry Worksheet With Answers Page 8
ADVERTISEMENT
anion. Stable anions are less reactive and are hence weaker bases. Since O is more
- is the strongest base in the set. In the remaining two
electronegative than N, the NH
2
COO - , the negative
species, the negative charge is on the O, but in the case of CH
3
charge is also delocalized by resonance.
10. D
11. See class notes, or p. 417 of the Wade textbook, for all the resonance structures of
the phenoxide ion shown below.
4 more resonance structures
O
(see p. 429 for resonance structures)
12. CH
< NH
< CH
OH < HCl
(periodic trend)
4
3
3
O - < H
N - Negatively charged ions are stronger bases than
13. H
O < NH
< CH
2
3
3
2
neutral counterparts. A table of pK
values will further aid in deciding the final order of
a
basicity.
14. B
15. B
16.
OH
H
C
CH
3
3
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4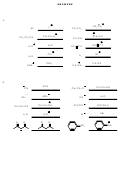 5
5 6
6 7
7 8
8








