Kinetics & Equilibrium Chemical Reactions Worksheet With Answer Key Page 10
ADVERTISEMENT
4. Remove H
Left
-----
Increases
Decreases
Remains
2
the same
5. Remove I
Left
Increases
-----
Decreases
Remains
2
the same
6. Remove HI
Right
Decreases
Decreases
-----
Remains
the same
7. Increase
Right
Decreases
Decreases
Increases
Increases
Temperature
8. Decrease
Left
Increases
Increases
Decreases
Decreases
Temperature
9. Increase
None
Remains
Remains
Remains
Remains
Pressure
the same
the same
the same
the same
10. Decrease
None
Remains
Remains
Remains
Remains
Pressure
the same
the same
the same
the same
9)
+
-
Stress
Equilibrium
Amount
[Na
]
[OH
]
K
Shift
NaOH(s)
1. Add
None
-----
Remains
Remains
Remains
NaOH(s)
the same
the same
the same
2. Add NaCl
Left
Increases
-----
Decreases
Remains
+
(Adds Na
)
the same
3. Add KOH
Left
Increases
Decreases
-----
Remains
-
(Adds OH
)
the same
+
4. Add H
Right
Decreases
Increases
-----
Remains
(Removes
the same
-
OH
)
5. Increase
Left
Increases
Decreases
Decreases
Decreases
Temperature
6. Decrease
Right
Decreases
Increases
Increases
Increases
Temperature
7. Increase
Left
Increases
Decreases
Decreases
Remains
Pressure
the same
8. Decrease
Right
Decreases
Increases
Increases
Remains
Pressure
the same
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8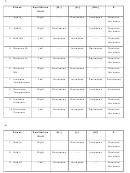 9
9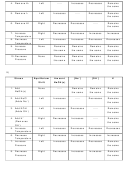 10
10 11
11








