Kinetics & Equilibrium Chemical Reactions Worksheet With Answer Key Page 4
ADVERTISEMENT
10. Decrease
Pressure
Le Chatelier’s Principle Continued
(g) ↔ 2HI(g)
ΔH = +12.6kcal
H
(g) + I
2
2
Stress
Equilibrium
[H
]
[I
]
[HI]
K
2
2
Shift
1. Add H
Right
-----
Decreases
Increases
Remains
2
the same
2. Add I
-----
2
3. Add HI
-----
4. Remove H
-----
2
5. Remove I
-----
2
6. Remove HI
-----
7. Increase
Temperature
8. Decrease
Temperature
9. Increase
Pressure
10. Decrease
Pressure
NaOH(s) ↔ Na
ΔH= -10.6kcal (remember that pure solids and liquids do not
+
-
(aq) + OH
(aq)
affect equilibrium values)
+
-
Stress
Equilibrium
Amount
[Na
]
[OH
]
K
Shift
NaOH(s)
1. Add
-----
NaOH(s)
2. Add NaCl
-----
+
(Adds Na
)
3. Add KOH
-----
-
(Adds OH
)
+
4. Add H
-----
(Removes
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8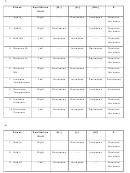 9
9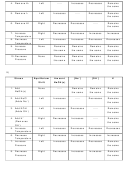 10
10 11
11








