Kinetics & Equilibrium Chemical Reactions Worksheet With Answer Key Page 3
ADVERTISEMENT
(a) Additional H
(g) is added to the equilibrium mixture at constant volume.
2
(b) The temperature of the equilibrium mixture is increased at constant volume.
(c)The volume of the container is decreased at constant temperature.
(d) The graphite pellets are pulverized.
Le Chatelier’s Principle
Le Chatelier’s Principle states that when a system at equilibrium is subjected to a stress, the
system will shift its equilibrium point in order to relieve the stress.
Complete the following chart by writing left, right, or none for equilibrium shift; and decreases,
increases, or remains the same for the concentrations of reactants and products, and for the
value of K.
(g) ↔ 2NH
ΔH = -22.0kcal
N
(g) + 3H
(g)
2
2
3
Stress
Equilibrium
[N
]
[H
]
[NH
]
K
2
2
3
Shift
1. Add N
Right
-----
Decreases
Increases
Remains
2
the same
2. Add H
-----
2
3. Add NH
-----
3
4. Remove N
-----
2
5. Remove H
-----
2
6. Remove
-----
NH
3
7. Increase
Temperature
8. Decrease
Temperature
9. Increase
Pressure
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8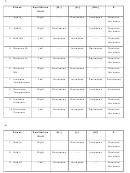 9
9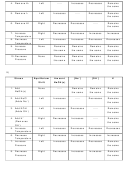 10
10 11
11








