Chemistry Unit C1 Worksheet - Study And Revision Pack (Foundation Paper) Page 18
ADVERTISEMENT
Revised
C1 Specification
1
2
3
4.1 Recall that:
a most metals are extracted from ores found in the Earth’s crust
b unreactive metals are found in the Earth as the uncombined elements
4.2 Describe how most metals are extracted from their ores by:
a heating with carbon, illustrated by iron
b electrolysis, illustrated by aluminium
4.3 Explain why the method used to extract a metal is related to its
position in the reactivity series and cost of the extraction process
4.4 Investigate methods for extracting a metal from its ore
4.5 Describe oxidation as the gain of oxygen and reduction as the
loss of oxygen
4.6 Recall that the extraction of metals involves reduction of ores
4.7 Recall that the oxidation of metals results in corrosion
4.8 Demonstrate an understanding that a metal’s resistance to
oxidation is related to its position in the reactivity series
4.9 Discuss the advantages of recycling metals, including economic
implications, and how recycling preserves both the environment
and the supply of valuable raw materials
4.10 Describe the uses of metals in relation to their properties,
including:
a aluminium
b copper
c gold
d steel
4.11 Use models to explain why converting pure metals into alloys
often increases the strength of the product
4.12 Demonstrate an understanding that iron is alloyed with other
metals to produce alloy steels with a higher strength and a greater
resistance to corrosion
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26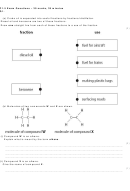 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34








