Chemistry Unit C1 Worksheet - Study And Revision Pack (Foundation Paper) Page 26
ADVERTISEMENT
5.29 Describe how bromine water is used to distinguish between
alkanes and alkenes
5.30 Describe how cracking involves the breaking down of larger
saturated hydrocarbon molecules (alkanes) into smaller, more
useful ones, some of which are unsaturated (alkenes)
5.31 Explain why cracking is necessary, including by using data
on the composition of different crude oils and the demand
for fractions in crude oil
5.32 Describe the cracking of liquid paraffin in the laboratory
5.33 Recall that:
a many ethene molecules can combine together in a
polymerisation reaction
b the polymer formed is called poly(ethene)
5.34 Describe how other polymers can be made by combining
together other monomer molecules, to include poly(propene),
poly(chloroethene) (PVC) and PTFE
5.35 Relate uses of the polymers poly(ethene), poly(propene),
poly(chloroethene) (PVC) and PTFE to the properties of the
compounds
5.36 Recall that most polymers are not biodegradable, that they
persist in landfill sites and that many produce toxic products
when burnt
5.37 Explain how some problems associated with the disposal of
polymers can be overcome:
a by recycling
b by developing biodegradable polymers
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26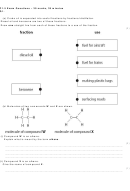 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34








