Chemistry Exam Worksheets With Answers - Chemistry 11, Exam Ii - 2006 Page 3
ADVERTISEMENT
4. Grab bag of multielectron atom questions.
a. Which element in the fourth row (K to Kr) would have the lowest second ionization
nd
+
++
-
energy and why? Remember: 2
Ionization Energy
Atom
------> Atom
+ e
+
1
2+
-
Ca
(4s
) to yield Ca
[Ar] + 1e
b. Which element in Group 17 (the halogens) would require the greatest energy photon to
remove its 1s electron. Why?
85
Element
At with 85 protons holds the 1s electron very strongly.
c. Which is the first element in which the n=4 shell is COMPLETELY filled?
70
14
Yb - when 4f
is achieved n=4 is completely filled.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6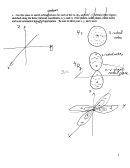 7
7 8
8 9
9 10
10 11
11 12
12