Chemistry Exam Worksheets With Answers - Chemistry 11, Exam Ii - 2006 Page 4
ADVERTISEMENT
Question II. Lewis Structures, VSEPR, dipoles (24)
Consider the following four molecules and molecular ions : POCl (phosporus monochlorine
monoxide); PH
(phosphine); POCl
(phosphorus trichlorine monoxide); PFCl
(phosphorus
3
3
4
monofluorine tetrachlorine):
On the following page:
1. Draw the best Lewis structure clearly indicating the total number of valence electrons, all bonding
and unshared electrons, and a formal charge for ALL atoms.
2. Next to the Lewis structure, sketch a VSEPR 3-D representation of the molecule. Indicate the
steric number of the central P atom (steric number is the number of bonded atoms plus lone pairs),
the geometry of the molecule, and the bond angles.
3. The electronegativites of P, O, Cl, H and F are 2.2, 3.4, 3.2, 2.2 and 4.0. Indicate on your VSEPR
sketch both individual bond dipoles (blue pen) and molecular dipoles (red pen) for each species.
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6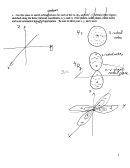 7
7 8
8 9
9 10
10 11
11 12
12