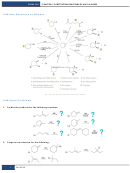
Writing And Balancing Chemical Reactions, Stoichiometry, Limiting Reactants Worksheet With Answers - Chem110 Page 2
ADVERTISEMENT
CHEM110 Week 5 Notes (Stoichiometry)
Page 2 of 8
__1__ Hg __1__
__1__ O __2__
The oxygen count is too low on the left side. A 2 coefficient before HgO fixes the O
count, but it causes the Hg count to be too low on the right side.
2 HgO(s)
→
Hg(l) + O
(g)
2
__2__ Hg __1__
__2__ O __2__
A 2 coefficient before Hg on the right side fixes the Hg count, and the reaction is
balanced.
2 HgO(s)
→
2 Hg(l) + O
(g)
2
__2__ Hg __2__
__2__ O __2__
Balance the following reaction:
P
(s) + O
(g) →
P
O
(s)
4
2
2
5
__4__ P __2__
__2__ O __5__
Sometimes balancing reactions is made easier by recognizing to start by balancing an
atom count with appropriate difficulty. In this example, starting in either place leads to
the same answer. For one approach, find the least common multiple for oxygen between 2
(left) and 5 (right), which is 10. To implement, add a 5 coefficient to O
on the left and a
2
2 coefficient to P
O
on the right. By doing so, the reaction is balanced.
2
5
P
(s) + 5O
(g) →
2P
O
(s)
4
2
2
5
__4__ P __4__
_10__ O _10__
Balance the following combustion reaction. A combustion reaction combines a fuel
(propane, C
H
) with an oxidant (O
). Combustion of a hydrocarbon (contains only
3
8
2
carbon and hydrogen) or a carbohydrate (carbon, hydrogen, and oxygen in a CH
O ratio)
2
produces carbon dioxide and water as products.
C
H
(g) + O
(g)
→
CO
(g) + H
O(g)
3
8
2
2
2
__3__ C __1__
__8__ H __2__
__2__ O __3__
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8