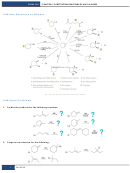
Writing And Balancing Chemical Reactions, Stoichiometry, Limiting Reactants Worksheet With Answers - Chem110 Page 7
ADVERTISEMENT
CHEM110 Week 5 Notes (Stoichiometry)
Page 7 of 8
Limiting Reactants
In some reactions, one of the reactants runs out first, and this limits the amount of product
that can form. Gasoline is the limiting reagent in your car (there is plenty of oxygen).
A related question about limiting reagents is if you had 3 tops and 11 legs in order to
build 3-legged stools. Which part would run out first?
Three tops (T) would require 9 legs (L) to make 3 stools (S), so the stool tops would run
out first. Two legs would be left over (in excess). In chemical terms T + 3L → S.
3T * (1S/1T) = 3S or 3 tops could produce 3 stools.
11L * (1S/3L) = 3.67S or 11 legs could produce 3.67 stools.
Because the tops produce fewer stools, the tops are the limiting reactant. To determine
how many legs are left over, do this calculation:
3T *(3L/1T) = 9L or 9 legs would be needed. The number of excess legs would be 11-9=
2 legs in excess.
With chemistry, we typically work in mass. We need to convert reactants to the mole to
count numbers (amounts) of reactants available and the numbers (amounts) of products
produced. Typically, we convert the amounts back to mass so we can use the balance.
If you mixed 5.0g of aluminum with 5.0g of oxygen, what mass of aluminum oxide
would form? How much excess reactant remains?
This is a limiting reagent question. The reagent that runs out first, the limiting reagent,
will produce the least product by grams or moles.
Step 1: Write the balanced chemical reaction
Step 2: Calculate moles of each reactant.
Step 3: Calculate product formed for each reactant. The limiting reactant makes the least
product. Pick a single product if more than one product is formed.
Step 1: 4Al(s) + 3O
(g) → 2Al
O
(s)
2
2
3
5.0 g Al
1 mol Al
2 mol Al
O
101.961 g Al
O
2
3
2
3
26.982 g Al
4 mol Al
1 mol Al
O
2
3
= 9.4g Al
O
formed (2SD).
2
3
5.0 g O
1 mol O
2 mol Al
O
101.961 g Al
O
2
2
2
3
2
3
31.998 g O
3 mol O
1 mol Al
O
2
2
2
3
= 11g Al
O
formed (2SD).
2
3
The reactant that forms the lesser quantity of product is the limiting reactant. Aluminum
is the limiting reactant because it forms only 9.4g of Al
O
, and this is the theoretical
2
3
yield of product that forms. The other reactant, O
, is in excess. To determine how much
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8