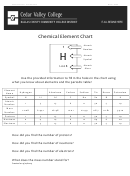
Chapter 2 An Introduction To Chemistry - The Structure Of Matter And The Chemical Elements Worksheet Page 21
ADVERTISEMENT
53
2.5 Common Elements
2.5
Common Elements
Most people look at a gold nugget and see a shiny metallic substance that can be
melted down and made into jewelry. A chemist looks at a substance such as gold and
visualizes the internal structure responsible for those external characteristics. Now that
we have discussed some of the general features of atoms and elements, we can return to
the model of solid, liquid, and gas structures presented in Section 2.1 and continue in
our quest to visualize the particle nature of matter.
Gas, Liquid, and Solid Elements
In Section 2.1, we pictured gases as independent spherical particles moving in straight‑
line paths in a container that is mostly empty space. This image is most accurate for the
o
24
bjeCtive
noble gases (He, Ne, Ar, Kr, Xe, and Rn): each noble gas particle consists of a single
atom. When we picture the helium gas in a helium‑filled balloon, each of the particles
in our image is a helium atom containing two protons and two neutrons in a tiny
nucleus surrounded by a cloud of negative charge generated by two electrons (Figure
2.12).
Figure 2.12
Helium Gas
o
24
bjeCtive
2 protons and
2 neutrons in
a tiny nucleus
2
He
−2 charge cloud
from 2 electrons
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7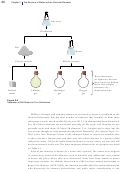 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35