Chapter 2 An Introduction To Chemistry - The Structure Of Matter And The Chemical Elements Worksheet Page 23
ADVERTISEMENT
55
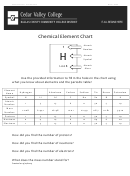
2.5 Common Elements
Because hydrogen molecules are composed of two atoms, they are called diatomic.
The elements nitrogen, oxygen, fluorine, chlorine, bromine, and iodine are also
o
26
bjeCtive
composed of diatomic molecules, so they are described as N
, O
, F
, Cl
, Br
, and I
.
2
2
2
2
2
2
Like the hydrogen atoms in H
molecules, the two atoms in each of these molecules
2
are held together by a covalent bond that is due to the sharing of two electrons.
Nitrogen, oxygen, fluorine, and chlorine are gases at room temperature and pressure,
so a depiction of gaseous N
, O
, F
, and Cl
would be very similar to the image of
2
2
2
2
H
in Figure 2.15.
2
Bromine, which is a liquid at room temperature, is pictured like the liquid shown in
Figure 2.2, except that each of the particles is a diatomic molecule (Figure 2.16).
Figure 2.16
o
24
bjeCtive
Liquid Bromine, Br
2
Each particle is a
diatomic molecule.
35
Br
Solid iodine consists of a very ordered arrangement of I
molecules. In order to
o
24
bjeCtive
2
give a clearer idea of this arrangement, the first image in Figure 2.17 on the next page
shows each I
molecule as a ball‑and‑stick model. The second image shows the close
2
packing of these molecules in the iodine solid. Remember that the particles of any
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35