Common Mathematical Operations In Chemistry Worksheets With Answers Page 11
ADVERTISEMENT
Appendix C
•
Equilibrium Constants for selected substances
A-11
Dissociation (Ionization) Constants (K
) of Selected Amine Bases
b
Name and Formula
Lewis Structure
†
K
K
b1
b2
Ammonia
1.76310
25
H
N
H
NH
3
H
H
H
C
C
Aniline
4.0310
210
H
C
C
N
H
C
H
NH
6
5
2
C
C
C
H
H
H
H
H
H
H
Diethylamine
8.6310
24
H
C
C
N
C
C
H
(CH
CH
)
NH
3
2
2
H
H
H
H
H
H
H
Dimethylamine
5.9310
24
H
C
N
C
H
(CH
)
NH
3
2
H
H
H
H
H
Ethanolamine
3.2310
25
H
O
C
C
H
N
HOCH
CH
NH
2
2
2
H
H
H
H
H
Ethylamine
4.3310
24
H
C
C
N
H
CH
CH
NH
3
2
2
H
H
H
H
H
Ethylenediamine
8.5310
7.1310
25
28
H
N
C
C
N
H
H
NCH
CH
NH
2
2
2
2
H
H
H
H
H
Methylamine
4.4310
24
C
H
N
H
CH
NH
3
2
H
H
H
H
N
H
H
4.8310
tert-Butylamine
24
C
C
C
H
H
(CH
)
CNH
3
3
2
H
H
C
H
H
H
H H
H
H
C
C
H
Piperidine
1.3310
23
C
N
H
C
H
NH
H
5
10
C
C
H
H
H H
H
H
H
3.5310
n-Propylamine
24
H
C
C
C
N
H
CH
CH
CH
NH
3
2
2
2
H
H
H
H
(continued )
†
Blue type indicates the basic nitrogen and its lone pair.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10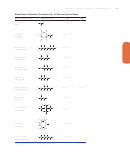 11
11 12
12 13
13 14
14








