Common Mathematical Operations In Chemistry Worksheets With Answers Page 9
ADVERTISEMENT
Appendix C
•
Equilibrium Constants for selected substances
A-9
Dissociation (Ionization) Constants (K
) of Selected Acids
a
Name and Formula
Lewis Structure
†
K
K
K
a1
a2
a3
H
O
O
H
O
H
Citric acid
7.4310
1.7310
4.0310
24
25
27
H
O
C
C
C
C
C
O
H
HOOCCH
C(OH)(COOH)CH
COOH
2
2
H
H
C
O
O
H
O
Formic acid
1.8310
24
H
C
O
H
HCOOH
H
H
O
Glyceric acid
2.9310
24
H
O
C
C
C
O
H
HOCH
CH(OH)COOH
2
H
H
O
H
O
Glycolic acid
1.5310
24
H
O
C
C
O
H
HOCH
COOH
2
H
H
O
Glyoxylic acid
3.5 310
24
O
C
O
C
H
HC(O)COOH
Hydrocyanic acid
6.2310
210
H
C
N
HCN
Hydrofluoric acid
6.8310
24
H
F
HF
Hydrosulfuric acid
9310
1310
28
217
H
S
H
H
S
2
Hypobromous acid
2.3310
29
H
O
Br
HBrO
Hypochlorous acid
2.9310
28
O
Cl
H
HClO
Hypoiodous acid
2.3310
I
211
H
O
HIO
O
Iodic acid
1.6310
I
21
O
O
H
HIO
3
O
H
H
Lactic acid
1.4310
24
H
C
C
C
O
H
CH
CH(OH)COOH
3
H
O
H
H
H
C
C
Maleic acid
1.2310
4.7310
22
27
O
C
C
O
HOOCCH CHCOOH
H
O
O
H
(continued )
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10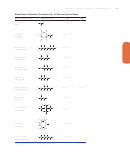 11
11 12
12 13
13 14
14








