Common Mathematical Operations In Chemistry Worksheets With Answers Page 8
ADVERTISEMENT
Appendix C
8
Chapter 1
•
Keys to the Study of Chemistry
Equilibrium Constants for Selected Substances*
Dissociation (Ionization) Constants (K
) of Selected Acids
a
Name and Formula
Lewis Structure
†
K
K
K
a1
a2
a3
H
O
Acetic acid
1.8310
25
C
H
H
C
O
CH
COOH
3
H
H
H
O
C
C
Acetylsalicylic acid
3.6310
24
H
C
C
C
O
H
CH
COOC
H
COOH
6
4
3
O
H
C
C
O
C
C
H
H
H
O
H
H
H
H
O
Adipic acid
3.8310
3.8310
25
26
H
O
C
C
C
C
C
C
O
H
HOOC(CH
)
COOH
2
4
H
H
H
H
O
Arsenic acid
6310
1.1310
3310
23
27
212
H
O
As
O
H
H
AsO
3
4
O
H
H
O
O
H
C
C
C
H
Ascorbic acid
1.0310
5310
25
212
H
C
C
O
H
C
H
O
O
2
6
6
6
O
C
C
H
H
H
H
H
H
O
C
C
Benzoic acid
6.3310
25
H
C
C
C
H
O
C
H
COOH
6
5
C
C
C
H
H
O
Carbonic acid
4.5310
4.7310
27
211
H
O
C
O
H
H
CO
2
3
H
O
Chloroacetic acid
1.4310
23
Cl
C
C
O
H
ClCH
COOH
2
H
Chlorous acid
1.1310
22
H
O
Cl
O
HClO
2
(continued)
*All values at 298 K, except for acetylsalicylic acid, which is at 37ºC (310 K) in 0.15 M NaCl.
†
Acidic (ionizable) proton(s) shown in red. Structures have lowest formal charges. Benzene rings show one resonance form.
A-8
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10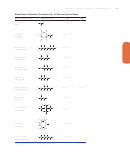 11
11 12
12 13
13 14
14








