Common Mathematical Operations In Chemistry Worksheets With Answers Page 12
ADVERTISEMENT
A-12
Appendix C
•
Equilibrium Constants for selected substances
Dissociation (Ionization) Constants (K
) of Selected Amine Bases
b
(continued )
Name and Formula
Lewis Structure
†
K
K
b1
b2
H
H
N
H
H
Isopropylamine
4.7310
24
C
C
C
H
H
(CH
)
CHNH
3
2
2
H
H
H
H
H
H
1,3-Propylenediamine
3.1310
3.0310
24
26
C
C
C
H
H
N
N
H
NCH
CH
CH
NH
2
2
2
2
2
H
H
H
H
H
H
H
C
C
Pyridine
1.7310
29
H
C
N
C
H
N
5
5
C
C
C
H
H
H
H
H
H
Triethylamine
5.2310
24
C
C
C
C
H
N
H
(CH
CH
)
N
3
2
3
H
H
H
C
H
H
H
C
H
H
H
H
H
Trimethylamine
6.3310
25
C
C
H
N
H
(CH
)
N
3
3
H
H
C
H
H
H
Dissociation (Ionization) Constants
Formation Constants (K
)
f
(K
) of Some Hydrated Metal Ions
of Some Complex Ions
a
Free
Complex Ion
K
f
Ion
Hydrated Ion
K
20
Ag(CN)
3.0310
a
2
2
Ag(NH
)
1.7310
7
Fe
31
Fe(H
O)
31
(aq)
6310
1
23
3
2
2
6
Ag(S
O
)
32
4.7310
13
21
21
Sn
Sn(H
O)
(aq)
4310
24
2
3
2
2
6
32
19
AlF
4 310
Cr
31
Cr(H
O)
31
(aq)
1310
24
6
2
6
Al(OH)
3 310
33
31
31
Al
Al(H
O)
(aq)
1310
2
25
4
2
6
Be(OH)
22
4 310
18
21
21
Cu
Cu(H
O)
(aq)
3310
28
4
2
6
22
6
CdI
1 310
Pb
21
Pb(H
O)
21
(aq)
3310
28
4
2
6
Co(OH)
22
5 310
9
21
21
Zn
Zn(H
O)
(aq)
1310
29
4
2
6
Cr(OH)
8.0310
29
Co
21
Co(H
O)
21
(aq)
2310
2
210
4
2
6
21
11
Cu(NH
)
5.6310
Ni
21
Ni(H
O)
21
(aq)
1310
210
3
4
2
6
Fe(CN)
42
3 310
35
6
Fe(CN)
32
4.0310
43
6
22
38
Hg(CN)
9.3310
4
Ni(NH
)
21
2.0310
8
3
6
Pb(OH)
8 310
13
2
3
25
Sn(OH)
3 310
2
3
Zn(CN)
22
4.2310
19
4
Zn(NH
)
21
7.8310
8
3
4
22
15
Zn(OH)
3 310
4
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10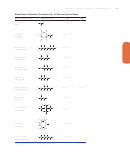 11
11 12
12 13
13 14
14








