Solubility Rules For Some Ionic Compounds In Water
ADVERTISEMENT
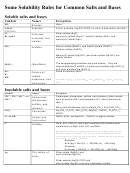
Solubility Rules for some ionic compounds in water
Soluble Ionic Compounds
+
+
+
1. All sodium (Na
), potassium (K
), and ammonium (NH
) salts are SOLUBLE.
4
–
–
–
–
2. All nitrate (NO
), acetate (CH
CO
), chlorate (ClO
), and perchlorate (ClO
) salts are SOLUBLE.
3
3
2
3
4
–
–
–
3. All chloride (Cl
), bromide (Br
), and iodide (I
) salts are SOLUBLE -- EXCEPT those also containing:
2+
+
2+
lead, silver, or mercury (I) (Pb
,Ag
, Hg
) which are NOT soluble.
2
2-
4. All sulfate (SO
) salts are SOLUBLE - - EXCEPT those also containing: calcium, silver, mercury (I),
4
2+
+
2+
2+
2+
2+
strontium, barium, or lead (Ca
, Ag
, Hg
, Sr
, Ba
, Pb
) which are NOT soluble.
2
Not Soluble Ionic Compounds
–
2–
5. Hydroxide (OH
) and oxide (O
) compounds are NOT SOLUBLE -- EXCEPT those also containing:
+
+
2+
sodium, potassium, or barium (Na
, K
, Ba
) which are soluble.
2–
6. Sulfide (S
) salts are NOT SOLUBLE -- EXCEPT those also containing: sodium, potassium,
+
+
+
2+
ammonium, or barium (Na
, K
, NH4
, Ba
) which are soluble.
2–
3–
7. Carbonate (CO
) and phosphate (PO
) salts are NOT SOLUBLE -- EXCEPT those also containing:
3
4
+
+
+
sodium, potassium, or ammonium (Na
, K
, NH
), which are soluble.
4
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1