Solubilities Of Inorganic Compounds In Water
ADVERTISEMENT
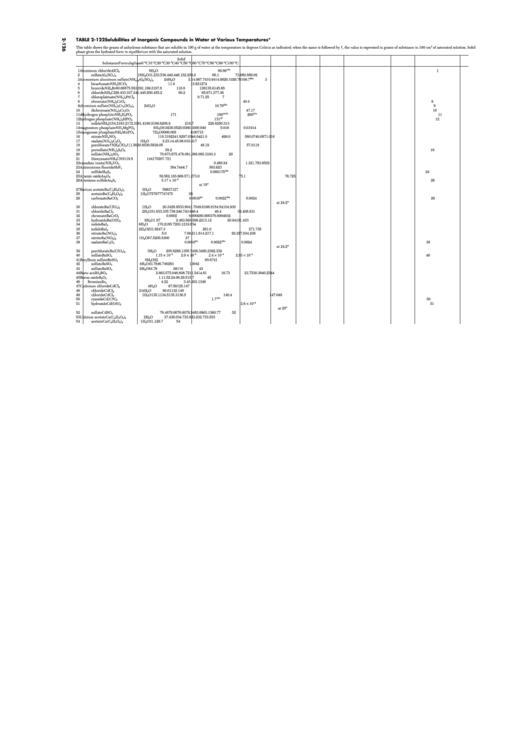
TABLE 2-122 Solubilities of Inorganic Compounds in Water at Various Temperatures*
This table shows the grams of anhydrous substance that are soluble in 100 g of water at the temperature in degrees Celsius as indicated; when the name is followed by †, the value is expressed in grams of substance in 100 cm
3
of saturated solution. Solid
phase gives the hydrated form in equilibrium with the saturated solution.
Solid
Substance
Formula
phase
0 °C
10 °C
20 °C
30 °C
40 °C
50 °C
60 °C
70 °C
80 °C
90 °C
100 °C
15
1
Aluminum chloride
AlCl
6H
O
69.86
°
1
3
2
2
sulfate
Al
(SO
)
18H
O
31.2
33.5
36.4
40.4
46.1
52.2
59.2
66.1
73.0
80.8
89.0
2
2
4
3
2
96
3
Ammonium aluminum sulfate
(NH
)
Al
(SO
)
24H
O
2.1
4.99
7.74
10.94
14.88
20.10
26.70
109.7
°
3
4
2
2
4
4
2
4
bicarbonate
NH
HCO
11.9
15.8
21
27
4
4
3
5
bromide
NH
Br
60.6
68
75.5
83.2
91.1
99.2
107.8
116.8
126
135.6
145.6
5
4
6
chloride
NH
Cl
29.4
33.3
37.2
41.4
45.8
50.4
55.2
60.2
65.6
71.3
77.3
6
4
7
chloroplatinate
(NH
)
PtCl
0.7
1.25
7
4
2
6
8
chromate
(NH
)
CrO
40.4
8
4
2
4
9
chromium sulfate
(NH
)
Cr
(SO
)
24II
O
10.78
25
9
°
4
2
2
4
4
2
10
dichromate
(NH
)
Cr
O
47.17
10
4
2
2
7
11
dihydrogen phosphite
NH
H
PO
171
190
14.5
260
31
11
°
°
4
2
3
12
hydrogen phosphate
(NH
)
HPO
131
15
12
4
2
4
13
iodide
NH
I
154.2
163.2
172.3
181.4
190.5
199.6
208.9
218.7
228.8
250.3
13
4
14
magnesium phosphate
NH
MgPO
6H
O
0.023
0.052
0.036
0.030
0.040
0.016
0.019
14
4
4
2
15
manganese phosphate
NH
MnPO
7H
O
0
0
0
0.005
0.007
15
4
4
2
16
nitrate
NH
NO
118.3
192
241.8
297.0
344.0
421.0
499.0
580.0
740.0
871.0
16
4
3
17
oxalate
(NH
)
C
O
1H
O
2.2
3.1
4.4
5.9
8.0
10.3
17
4
2
2
4
2
18
perchlorate†
NH
ClO
†
11.56
20.85
30.58
39.05
48.19
57.01
18
4
4
19
persulfate
(NH
)
S
O
58.2
19
4
2
2
8
20
sulfate
(NH
)
SO
70.6
73.0
75.4
78.0
81.0
88.0
95.3
103.3
20
4
2
4
21
thiocyanate
NH
CNS
119.8
144
170
207.7
21
4
22
vanadate (meta)
NH
VO
0.48
0.84
1.32
1.78
3.05
22
4
3
23
Antimonious fluoride
SbF
384.7
444.7
563.6
23
3
18
24
sulfide
Sb
S
0.000175
°
24
2
3
25
Arsenic oxide
As
O
59.5
62.1
65.8
69.5
71.2
73.0
75.1
76.7
25
2
5
26
Arsenious sulfide
As
S
5.17 × 10
−5
26
2
3
at 18°
27
Barium acetate
Ba(C
H
O
)
3H
O
59
63
71
27
2
3
2
2
2
28
acetate
Ba(C
H
O
)
1H
O
75
79
77
74
74
75
28
2
3
2
2
2
8
18
29
carbonate
BaCO
0.0016
°
0.0022
°
0.0024
29
3
at 24.2°
30
chlorate
Ba(ClO
)
1H
O
20.34
26.95
33.80
41.70
49.61
66.81
84.84
104.9
30
3
2
2
31
chloride
BaCl
2H
O
31.6
33.3
35.7
38.2
40.7
43.6
46.4
49.4
52.4
58.8
31
2
2
32
chromate
BaCrO
0.0002
0.00028
0.00037
0.00046
32
4
33
hydroxide
Ba(OH)
8H
O
1.67
2.48
3.89
5.59
8.22
13.12
20.94
101.4
33
2
2
34
iodide
BaI
6H
O
170.2
185.7
203.1
219.6
34
2
2
35
iodide
BaI
2H
O
231.9
247.3
261.0
271.7
35
2
2
36
nitrate
Ba(NO
)
5.0
7.0
9.2
11.6
14.2
17.1
20.3
27.0
34.2
36
3
2
37
nitrite
Ba(NO
)
1H
O
67.5
205.8
300
37
2
2
2
8
18
38
oxalate
BaC
O
0.0016
°
0.0022
°
0.0024
38
2
4
at 24.2°
39
perchlorate
Ba(ClO
)
3H
O
205.8
289.1
358.7
426.3
495.2
562.3
39
4
2
2
−4
−4
−4
−4
40
sulfate
BaSO
1.15 × 10
2.0 × 10
2.4 × 10
2.85 × 10
40
4
41
Beryllium sulfate
BeSO
6H
O
52
60.67
41
4
2
42
sulfate
BeSO
4H
O
43.78
46.74
62
83
100
42
4
2
43
sulfate
BeSO
2H
O
84.76
98
110
43
4
2
44
Boric acid
H
BO
2.66
3.57
5.04
6.60
8.72
11.54
14.81
16.73
23.75
30.38
40.25
44
3
3
45
Boron oxide
B
O
1.1
1.5
2.2
4.0
6.2
9.5
15.7
45
2
3
46
Bromine
Br
4.22
3.4
3.20
3.13
46
2
47
Cadmium chloride
CdCl
4H
O
97.59
125.1
47
2
2
48
chloride
CdCl
2aH
O
90.01
132.1
48
2
2
49
chloride
CdCl
1H
O
135.1
134.5
135.3
136.5
140.4
147.0
49
2
2
50
cyanide
Cd(CN)
1.7
15
50
°
2
51
hydroxide
Cd(OH)
2.6 × 10
−4
51
2
at 25°
52
sulfate
CdSO
76.48
76.00
76.60
78.54
83.68
63.13
60.77
52
4
53
Calcium acetate
Ca(C
H
O
)
2H
O
37.4
36.0
34.7
33.8
33.2
32.7
33.5
53
2
3
2
2
2
54
acetate
Ca(C
H
O
)
1H
O
31.1
29.7
54
2
3
2
2
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4








