Rrevision Guide Organic Analysis Page 3
ADVERTISEMENT
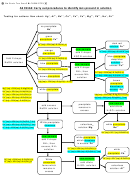
Identification of Functional Groups by test-tube reactions
Functional group
Reagent
Result
Alkene
Bromine water
Orange colour
decolourises
Aldehyde
Fehlings solution
Blue solution to red
precipitate
Aldehyde
Tollens Reagent
Silver mirror formed
Carboxylic acid
Sodium carbonate
Effervescence of CO
2
evolved
o
o
1
2
alcohol and
Sodium dichromate and
Orange to green colour
aldehyde
sulphuric acid
change
chloroalkane
Warm with silver nitrate
Slow formation of white
precipitate of AgCl
Tollen’s Reagent
Fehling’s solution
2+
Reagent: Fehling’s Solution containing blue Cu
ions.
Reagent: Tollen’s Reagent formed by mixing aqueous
ammonia and silver nitrate. The active substance
Conditions: heat gently
+
is the complex ion of [Ag(NH
)
]
.
Reaction: aldehydes only are oxidised by Fehling’s
3
2
Solution into a carboxylic acid and the copper (II)
Conditions: heat gently
ions are reduced to copper(I) oxide .
Reaction: aldehydes only are oxidised by Tollen’s
2+
Observation: Aldehydes :Blue Cu
ions in solution
reagent into a carboxylic acid and the silver(I)
change to a red precipitate of Cu
O. Ketones do
2
ions are reduced to silver atoms
not react
Observation: with aldehydes, a silver mirror forms
coating the inside of the test tube. Ketones result
2+
+
CH
CHO + 2Cu
+ 2H
O
CH
COOH + Cu
O + 4H
3
2
3
2
in no change.
+
+
CH
CHO + 2Ag
+ H
O
CH
COOH + 2Ag + 2H
3
2
3
The presence of a carboxylic acid can be tested by
addition of sodium carbonate. It will fizz and produce
carbon dioxide
-
+
2CH
CO
H + Na
CO
2CH
CO
Na
+ H
O + CO
3
2
2
3
3
2
2
2
N Goalby
3
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3