Extraction Of Metals Page 2
ADVERTISEMENT
4/26/2010
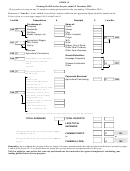
How does the reactivity affects the extraction?
potassium
Extracted by
using
sodium
Answer
electricity
to decompose
calcium
the molten metal compounds
magnesium
The positioning of a metal in the
(electrolysis)
aluminium
(carbon)
reactivity series will determine the
zinc
iron
Extracted by
reducing the
method used for its extraction.
lead
metal oxides using carbon
copper
(reduction)
silver
Found
naturally
gold
uncombined as metals
The more reactive the metal is, the harder it is to extract the metal from its ores.
What is Reduction?
Exercise
Metals are often found combined with oxygen as oxides.
To obtain the metal, the oxygen must be removed.
The removal of oxygen from a substance is called reduction.
reduction
metal oxide (in ore)
metal
Carbon can be used to extract metals by reduction.
2PbO + C
2Pb + CO
2
In this reaction, the carbon removes oxygen from lead oxide.
This occurs because
carbon is more reactive than
lead.
• The main ore of iron is haematite.
Extracting iron from Haematite
• Haematite contains
iron (III) oxide
mixed
with impurities such as sand and clay.
• Iron is extracted from haematite in a
blast
Main ore of iron
furnace.
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4