Chemistry Worksheet Page 4
ADVERTISEMENT
CHE107-003
Practice Exam 3 Key
9. What is the total number of electrons possible in the 2p orbitals?
6
10. (Multiple choice) The general electron configuration for the outer-shell
electrons of noble gas atoms is:
2
6
A. ns
np
2
5
B. ns
np
2
4
C. ns
np
2
3
D. ns
np
6
E. nsnp
2-
11. (Multiple choice) The sulfide ion, S
, is isoelectronic with which one of the
following species?
2-
A. O
-
B. F
+
C. Na
3+
D. Al
+
E. K
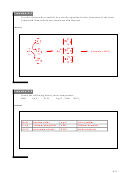
12. Write the ground-state electron configuration of a neon (Ne) atom. Refer
to Figure 7.21, if necessary.
2
2
2
1s
2s
or [He]2s
or use an orbital diagram
4
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4