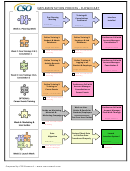
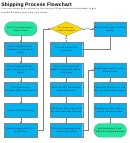
Research Process Flowchart Page 12
ADVERTISEMENT
6. Obtain Ethical and Trust approval?
COREC -
Central Office for Research Ethics Committees
Do you know where to start and what to consider in relation to ethics in
your research project?
The Central Office for Research Ethics Committees (COREC):
co-ordinates the development of operational systems for local and
multi-centre Research Ethics Committees (LRECs and MRECs), on
behalf of the National Health Service in England;
maintains an overview of the operation of the research ethics system
in England, and alerts the Department of Health and other
responsible authorities if the need arises for them to review policy
and operational guidance relating to Research Ethics Committees
(RECs);
manages the MRECs in England;
develops and manages a national training programme for REC
members and administrators in England;
maintains close contact with officials in the Department of Health
with policy responsibility for wider issues of research ethics and with
colleagues from Northern Ireland, Scotland and Wales;
with appropriate advice, develops, implements and maintains
operating procedures and standards for RECs that will be consistent
across the UK;
establishes and manages regional Offices of Research Ethics
Committees (ORECs) to oversee the activity of LRECs;
provides advice to the Department of Health on the implications and
practicalities of transposing the European Clinical Trials Directive in
the UK.
Know your Ethics Committee
Research Ethics Committees (RECs) have been working to standard
operating procedures since 1st March 2004, and the COREC website gives
people access to comprehensive and up-to-date information on the REC
system in the UK including:
New Operational Procedures for NHS Research Ethics Committees:
Guidance for Applicants to RECs
When to apply for Ethical Approval
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Business
 1
1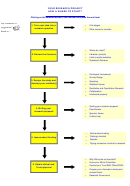 2
2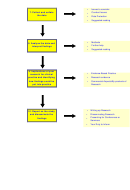 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22