Chemistry Worksheets Page 2
ADVERTISEMENT
Answers to Worksheet # 5
Molecules, Compounds, and Formulas
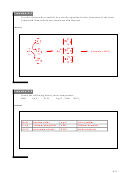
1. A molecular formula has the actual number of atoms, but has no information on bonding or
structure. An example of a molecular formula would be C
H
O. A condensed formula
3
8
indicates how the atoms are grouped together. For the molecular formula C
H
O, the
3
8
condensed formula might be CH
CH
CH
OH. A structural formula has the actual number of
3
2
2
atoms as well as the bonding between them.
Ionic Compounds
2. Metals usually form cations, or positive ions, by losing electrons. Nonmetals usually form
anions, or negative ions, by gaining electrons (with some exceptions). A cation is positive,
while an anion is negative.
3. Mg is a metal in group II, so, like main group metals, it will lose electrons to have a charge
2+
equal to its group number. Mg will lose 2 electrons to become Mg
, which is a cation.
4. Cl is a nonmetal in group VII, so its charge, like most main group nonmetals, will be the
group number – 8, or 7 – 8 = -1, which is an anion.
5. A list of polyatomic ions appears on page 107 in table 3.1. You must memorize the charge,
2-
name and formula of the polyatomic ions. a) CaCO
contains CO
,which is carbonate; b)
3
3
-
H
O contains OH
, which is hydroxide; c) NaCl does not have a polyatomic ion in it; d)
2
3-
2-
Na
PO
contains PO
, which is phosphate; e) CH
CO
H contains CH
CO
, which is acetate
3
4
4
3
2
3
6. When an ionic bond forms, the metal donates an electron to the nonmetal. The attraction of
opposite charges keeps the ions together in an ionic bond.
Binary Ionic Compound Naming
When naming binary (two elements) ionic compounds, always write the name of the cation first.
With Main Group Elements:
The cation keeps its name and the anion changes its ending to ide. The name of the cation and
+
the anion are two separate words (example: NaCl. Na
is the cation; it keeps its name of sodium.
-
Cl
is the anion; it changes its ending from chlorine to chloride. The name of NaCl is sodium
chloride).
With Transition Metals:
Anion naming is the same as above. Note the charge of the cation in parentheses in roman
numerals since these charges change. The name of the cation and its roman numerals are one
-
word, the anion name is a separate word (example: CuCl. Cl
is the anion. Since only one Cu is
needed to balance it, Cu must have a charge of +1. Therefore, CuCl is named copper(I) chloride).
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3